FOCUS: IRON OVERLOAD
Hereditary hemochromatosis (HH) is a disorder of iron regulation that leads to excessive iron absorption. Over time, the resultant iron overload and deposition in tissue leads to various chronic diseases and premature death. Even though it is the most common genetic disorder among Caucasians in the U.S., hereditary hemochromatosis often goes undetected or unrecognized by healthcare providers. Laboratory tests provide effective, inexpensive means of screening for and confirming hereditary hemochromatosis. The clinical laboratory also plays a key role in hereditary hemochromatosis treatment, reduction of iron stores through therapeutic phlebotomy.
ABBREVIATIONS: CAP = College ofAmerican Pathologists; GI = gastrointestinal; HFE = hemochromatosis gene; HH hereditary hemochromatosis; HII = hepatic iron index; JH = juvenile hemochromatosis; SF = serum ferritin; Tf = transferrin; Tfr = transferrin receptors; TS = transferrin saturation.
INDEX TERMS: hemochromatosis.
Clin Lab Sci 2001; 14(3):196
CASE STUDY
A 56-year-old Caucasian woman saw her family physician for her annual physical. She states she is in generally good health but complains of fatigue, mild depression, and mild to moderate joint pain of several months duration. She holds a full-time job as an accountant. Her medical history is significant only for hormone replacement therapy for the past 12 years. She has three children, ages 30, 26, and 17 years. The patient states that her consumption of alcohol is limited to one to two glasses of wine each week. She takes ibuprofen occasionally for joint pain but denies use of any other over-the-counter medication except daily vitamin tablets. Family history reveals that her father died ten years ago of liver disease at age 70. The patient is uncertain about the exact nature of her father's illness. Her mother is alive and in good health. She also states that she has two siblings, a sister who was recently diagnosed with diabetes and a brother. Physical exam reveals a well-- appearing female with normal vital signs. There is a mild hepatomegaly and joint tenderness. Laboratory studies were ordered on a fasting specimen and are reported in Table 1. Continuation and discussion of the case are found at the conclusion of the article.
Hereditary hemochromatosis (HH) is an autosomal recessive disorder of iron regulation that results in excessive dietary iron absorption through the gastrointestinal tract. Over time, the resultant iron overload and its deposition in tissue leads to hepatic cirrhosis and cancer, diabetes mellitus, cardiomyopathy, arthritis, hypogonadism, other chronic disorders, and death. Although it is a genetic disorder, clinical symptoms most typically become apparent in homozygous individuals in middle age.
A gene associated with HH was identified in 1996.(1) Results of recent studies indicate that HH is the most common genetic disorder in North America and that it exists in approximately two to five per 1000 Caucasian Americans.2,3 Between 10% and 15% of the US population may be heterozygous carriers of HH.4,5 While HH is most prevalent among persons of Northern European origin and descent, it is also found in other ethnic groups.6 Hemochromatosis is more common than other genetic disorders with which there is greater familiarity, e.g., cystic fibrosis and sickle cell disease.
Due to lack of awareness, HH often goes undetected or unrecognized by healthcare providers.7 Because symptoms have a gradual onset, are nonspecific, and mimic other common disorders, diagnosis and treatment are often delayed, leading to increased morbidity and mortality. Laboratory tests that assess iron levels, particularly transferrin saturation, provide effective means of screening individuals for HH and are recommended by the College of American Pathologists (CAP). Genetic testing is available, but is not recommended by the CAP for population screening at this time.8 Persons with elevated iron parameters on laboratory screening tests can be referred for medical evaluation and confirmatory testing, including genetic tests, for the common mutations associated with HH. Treatment for HH, removal of stored iron through therapeutic phlebotomy, is effective, safe, and economical.
It is estimated that as many as 1.5 million persons in the US may have iron overload disorders. Primary iron overload due to HH is the most common cause.9 Many cases of HH remain unrecognized. Early detection to prevent the serious complications associated with HH will therefore have important consequences for reducing morbidity and mortality for a substantial number of individuals.
NORMAL IRON METABOLISM
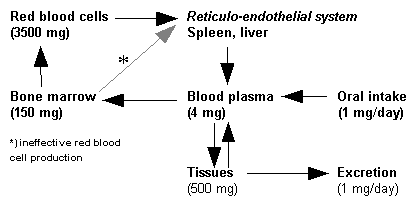
Iron is needed by all body cells and is crucial for oxygen transport, oxidative metabolism, and cell growth and proliferation. To serve these functions, iron must be bound to protein. Iron is potentially harmful when ionized or complexed to inorganic compounds.10 Two types of iron-containing compounds are normally found in the body: compounds that serve in metabolic or enzymatic functions and storage compounds. Hemoglobin, myoglobin, cytochromes, and other proteins are involved in oxygen transport and utilization. Iron in hemoglobin (heme iron) comprises 65% to 80% of total body iron.11
Storage compounds, including ferritin and hemosiderin, are the second type of iron-containing compound. Ferritin is a proteinbound, water-soluble, mobilizable storage compound and is the major source of storage iron. Hemosiderin is a water-insoluble form that is less readily available for use. When the amount of total body iron is relatively low, storage iron consists predominately of ferritin. When iron stores are high, hemosiderin predominates. Unlike ferritin, hemosiderin stains with the Prussian blue stain (Pens reaction) and may be observed in tissues. Storage forms normally comprise approximately 30% of total body iron. Iron stores provide a source of iron when physiologic demand is high, e.g., blood loss, pregnancy, and periods of rapid growth.11
The typical daily diet of most North Americans contains approximately 15 mg of iron. The normal individual absorbs only 5% to 10% of dietary iron, or about one to two mg daily. Furthermore, iron is well conserved, with heme iron from senescent RBCs being cycled back into the iron pool and reused for incorporation in developing erythrocytes, Iron is lost from the body only in small amounts, primarily through desquamation of mucosal cells in the gastrointestinal (GI) tract and losses through body secretions, including urine, sweat, and feces.11
Regulation of iron equilibrium occurs through the process of absorption.11,12 Factors affecting absorption include:
* condition of GI tract mucosal cells
* intraluminal factors, e.g. motility
* dietary intake, including form of iron ingested, e.g., heme iron is more readily absorbed than non-heme forms
* tissue stores, e.g., decreased storage iron increases absorption
* rate of hematopoietic activity, e.g., increased rate increases absorption
* oxygen concentration in tissues, e.g., hypoxia increases absorption
Once absorbed through the mucosal cells of the duodenum, iron is bound to a carrier protein for movement to sites of utilization. Almost all iron in plasma is bound to transferrin (Tf), and at least 80% of Tf-bound iron is carried to the bone marrow.12 Tf is normally about one-third saturated with iron. Transferrin releases iron to specific transferrin receptors (TfR) for movement into cells. TfR are found on all cells but are found in relatively high concentration in erythroid precursors, hepatocytes, and placental cells. When the capacity of plasma Tf to bind iron is exceeded, i.e., transferrin saturation (TS) is higher than normal, excess iron is taken up by hepatocytes and other cells.13
PATHOPHYSIOLOGY
Hemochromatosis is an autosomal recessive genetic disorder characterized by iron overload. Persons homozygous for mutations of the hemochromatosis gene, HFE, absorb three to four mg of dietary iron daily, a rate two to three times greater than normal. Normal storage sites become overloaded with iron, resulting in ferritin levels as much as ten times normal.12 With no mechanism to dispose of the excess, iron is deposited in the parenchymal cells of the liver, pancreas, pituitary, heart, synovium, and other tissues. Iron in excess of normal cellular ferritin stores contributes to the generation of free radicals and reactive oxygen intermediates that cause cell damage to organs and tissues.10,12
The amount of time needed for iron to increase to levels causing organ damage is variable and most likely partially dependent on gender and dietary factors. Blood loss through menstruation and pregnancy are thought to delay the onset of iron overload, and therefore symptoms, in women. Similarly, regular blood donation may confer some degree of protection. As levels of storage iron increase, clinical features of iron overload, including hepatic dysfunction or failure, diabetes, hypogonadism, arthritis, cardiomyopathy. hyperpigmentation, and fatigue, may become evident. Symptomatic patients typically present in middle age between the ages of 30 and 60, although this is quite variable. Persons as young as 20 may show clinical signs and symptoms of HH.14
GENETICS
The hemochromatosis gene was identified by positional cloning in 1996.1 The locus for the gene is on the long arm of chromosome 6 where it codes for a membrane protein, HFE, which is 343 amino acids in size.15 The exact mechanism of the role of HFE in iron metabolism is not completely understood. A popular hypothesis is that HFE, along with a second protein, beta-2 microglobulin (beta-2M), interacts with TfR on cell membranes. This interaction represses the affinity of Tf for the receptor, thus lowering the uptake of Tf into the cell. TfR have been found on the surface of a variety of cells, with the greatest concentration on the basilateral membrane of the intestine, hepatocytes, and erythrocyte precursors.16
In HH, two mutations of the HFE gene have been described, C282Y and H63D. In the more prevalent C282Y mutation, there is a base substitution leading to a change in the amino acid in position 282 from cysteine (C) to tyrosine (Y) (Figure 1). The loss of the sulfhydryl-containing amino acid disrupts the tertiary structure of HFE so that it no longer binds to beta-2 M. beta-2M appears to act along with other proteins to chaperone the newly synthesized HFE out of the Golgi and to the cell surface where it can then bind to TfR. In the C282Y mutation, HFE remains in the Golgi, never making it to the cell surface. The result is that Tf binding to TfR is enhanced and excessive amounts of iron are absorbed in the small intestine, hepatocytes, and other tissues.1,16,17
The second mutation, H63D, occurs when a histidine (H) residue in position 63 is replaced by an aspartic acid (D) (Figure 1). The mechanism by which this mutation leads to increased iron uptake is less well understood when compared to the C282Y mutation. Unlike the C282Y mutation, the H63D mutation does not seem to affect the binding of beta-2M and intracellular trafficking, since detectable concentrations of the mutated protein are found on cell membranes. Some researchers speculate that the H63D mutation affects the binding of proteins involved in iron regulation and uptake at the cell surface. 17 A simplified theoretical model diagramming effects of the two mutations of HFE is shown in Figure 2.
EPIDEMIOLOGY
The prevalence of common HFE mutations among persons with HH has been reported in several recent studies. Results of studies conducted in the US, France, and Australia are summarized in Table 2. Homozygous C282Y mutation is present in 82% to 100% of patients with HH, suggesting a strong link between this genotype and the presence of clinical disease.1,18,19,20
A small percentage of persons with HH do not have two C282Y mutations. Results of three studies indicate that 4% to 5% of persons with HH are compound heterozygotes for C282Y and H63D. Some, 1% to 2%, hemochromatosis patients are homozygous for the H63D mutation, and 3% to 4% are heterozygous for this mutation. Hemochromatosis is also reported in 0% to 1% of persons who are heterozygous for the C282Y mutation.1,18,19 Neither mutation was present in 7% of HH patients in two studies.1,18 Iron overload may have actually been attributable to other unrecognized causes in subjects in some studies.
Within the Caucasian population 10% to 15% are heterozygote carriers of the C282Y mutation, and approximately 25% are carriers of a single H63D mutation. For reasons yet unknown, not all individuals who are homozygous for the C282Y mutation display phenotypic features of HH, and most patients with H63D polymorphisms do not develop iron overload. Results of two studies assessing prevalence of HFE mutations in normal controls are reported in Table 3.1,18 As many as 32 million Americans may be carriers of a single gene. It has been estimated that at least 25% of females and 50% of males who are genetically diagnosed as HH will develop the signs and symptoms of iron overload. Conversely, 20% to 25% of C282Y heterozygotes manifest phenotypic features of HH as evidenced by elevated transferrin saturation, but most do not appear to have the significant organ damage associated with homozygote mutations.7 It may be that symptomatic heterozygotes are actually HFE-compound heterozygotes with the second unidentified mutation modifying the expression of the more severe known mutation. It is quite likely that more mutations of HFE and elucidation of other gene mutations linked with HFE will be discovered in the near future, enabling scientists to better explain the phenotypic heterogeneity of this disorder.
The C282Y mutation is thought to have arisen 60 to 70 generations ago and has persisted due to its possibly conferring an early survival advantage to affected persons, primarily females.21 The presence of the gene may have promoted increased iron absorption at a time in which diets were iron deficient. Typical diets in developed countries today supply sufficient iron to meet nutritional requirements of most of the population. Only in recent decades has the average life expectancy increased sufficiently that affected individuals live long enough to accumulate iron and die of HH.
HH appears to be most prevalent among people in the Northern British Isles-Scotland, Ireland, and Great Britain-who are of Celtic descent. Studies in Ireland have found the allele frequency to be 14% for the C282Y mutation, suggesting a Celtic origin for the hemochromatosis gene.22,23 Persons of Celtic descent in other geographic areas, e.g., United States, Australia, and France also have a relatively high incidence of HH. Relatively high incidence of the mutation has also been found among Danes, Icelanders, Norwegians, and Bavarians.6
The incidence of iron overload as measured by transferrin saturation in the U.S. is much higher in Caucasians than in African Americans, although the incidence in Hispanics is similar to that of Caucasians. The frequency of the C282Y mutation in non Caucasian populations in the U.S. has yet to be adequately determined.
DIAGNOSIS
Early identification of persons with HH is essential to prevent serious and irreversible complications associated with severe iron overload. Although rarely seen in clinical practice, the classic triad of skin hyperpigmentation (bronzing), type 2 diabetes, and hepatic cirrhosis has long been recognized as evidence of advanced iron overload. Persons with HH typically present with a much wider variety of signs and symptoms, particularly if they are seen before significant iron accumulation has occurred. Age at presentation and disease severity are highly variable.
HH is frequently discovered only during management of associated illness or routine health examinations. It has been estimated that only 10% of affected persons are actually being diagnosed at the present time." Under-diagnosis of HH is thought to occur due to:
* Lack of specificity of signs and symptoms
* Asymptomatic status of affected persons until damage to organs and tissues has occurred
* Confusion with liver disease due to other causes
* Insufficient awareness and knowledge of HH
Diagnosis of persons suspected of having iron overload depends on demonstrating excess iron in blood or tissues. A diagnostic laboratory algorithm for hemochromatosis testing is shown in Figure 3.
Upon diagnosis of HH, relatives of an affected person should also be tested using tests for iron andlor DNA testing. It is possible that over the next few years population screening for HH will become routine, resulting in detection prior to development of symptoms. Clinical signs and symptoms
Early signs and symptoms of HH are nonspecific and variable. As iron overload progresses, signs and symptoms of organ damage become more severe. Early and late signs and symptoms are listed in Table 4.(7,25)
Laboratory tests for HH
Laboratory tests for assessing iron status and diagnosing HH are found in Table 5. Serum iron (SI) is a measure of the circulating iron bound to Tf and is reflective of total body iron. SI is elevated in HH and acute hepatitis and is decreased in iron deficiency anemia and chronic inflammation. SI concentrations exhibit diurnal variation, with the lowest values occurring around midnight. In addition, specimens collected from the same individual at the same time of the day can have day to day variations as high as 40%.26 SI results are also affected by diet, menstrual cycle, pregnancy, ingestion of iron supplements, and oral contraceptive use. SI levels alone are insensitive indicators of HH.8
Serum is the specimen of choice for SI, but if plasma is used, anticoagulants such as EDTA and citrate that chelate iron must be avoided. SI is measured on automated analyzers employing a spectrophotometric method. Iron in the sample is released from transferrin with an acid reagent, reduced to the ferrous state, and reacted with a chromogen such as bathophenanthroline, Ferene(R), or ferrozine. The intensity of the color change is proportional to the iron concentration. Interference can arise from the use of a hemolyzed sample and contamination of reagents and water with iron. Reference intervals for SI vary according to methodology, sex, and age and should be determined independently by each laboratory.
Tests for Tf measure the concentration of the primary carrier protein for iron and are typically performed along with the SI. Taken together, these determinations are useful in the differential diagnosis of many disorders affecting iron metabolism, including HH. Tf is decreased in HH and increased in iron deficiency. Serum Tf can be measured directly using immunochemical methods such as nephelometry and turbidimetry.
Measuring total iron binding capacity (TIBC) is an indirect method of assessing Tf and provides essentially equivalent information. TIBC is performed by adding Fe^sup +3^ in sufficient quantity to the specimen to completely fill all iron binding sites on Tf. Excess unbound iron is removed by adsorption with magnesium carbonate, alumina, or ion resin. The iron content of the saturated binding protein is then measured as described above.
Using the Sl and TIBC values, the percentage of transferrin saturated with iron is calculated using the formula:
Transferrin saturation (TS) =SI/TIBC x 100
The reference interval for TS is typically 20% to 55% for males and 15% to 50% for females. TS is the most sensitive laboratory test for the diagnosis of HH and will be elevated prior to significant deposition of tissue iron. TS of >60% on two occasions is considered suggestive of HH by the College of American Pathologists (CAP).8 Others recommend much lower TS levels as cause for further testing. A fasting TS of >45% will identify 98% of persons with HH with relatively few false positives.27 TS levels may be affected by diurnal variation, dietary factors, and coexisting disease states such as inflammation and hepatitis. Patients with HH can have a falsely normal TS if chronic blood loss or inflammatory disease is present.8 Patients with initially increased TS should be followed by performing a second TS from a morning fasting specimen. The patient should also be advised not to take vitamins supplemented with iron or oral contraceptives for several days prior to the repeated test.28
Serum ferritin (SF) level reflects the amount of storage iron in tissues and is measured in serum using immunochemical methods such as ELISA, IRMA, and immunofluorometry. As with SI, each laboratory should determine its own method specific reference intervals. Upper limits of reference intervals for SF are 200 ng/mL for premenopausal women and 300 ng/mL for men and post-menopausal women. An elevated SF combined with elevated TS implies primary iron overload. Patients with HH generally show increases in SF as young adults, but a normal SF does not rule out the diagnosis of the disease, particularly in children and females.15 SF alone is inadequate as a screening test because it lacks the necessary sensitivity and specificity. SF is frequently elevated in persons with inflammation, cancer, or infection. The CAP recommends performing the SF following the finding of an elevated TS.8
Laboratory tests assessing iron metabolism should be interpreted with caution since a number of pre-analytical and physiological issues can affect the results. (Table 6). For a comprehensive review of performance characteristics of the various laboratory tests used to diagnose HH the reader is referred to Witte.8
Direct DNA tests for the 2 genetic mutations associated with HH are available. DNA is extracted from whole blood samples collected in ACD or EDTA tubes, and the region surrounding the two mutations is amplified by polymerase chain reaction (PCR) using specific oligonucleotide primers. The PCR products are digested with restriction enzymes, gel electrophoresed, and stained with ethidium bromide, resulting in DNA fragment sizes unique for each mutation. The gels are visually inspected and interpreted by comparison to normal controls and size markers. Using RsaI restriction enzymes along with primers for the C282Y mutation, the mutation creates an additional restriction recognition site. With this technique, persons having the C282Y allele will have a smaller DNA fragment when compared to persons with the normal FIFE allele (Figure 4).29 DNA testing is most useful in confirmatory testing of individuals with documented iron overload (increased TS and SF), in testing pre-symptomatic individuals (increased TS, normal SF and liver function tests), and in screening HH family members. DNA testing can not predict severity of the disorder, and a negative test result does not rule out a diagnosis because of genetic heterogeneity and lack of phenotypic expression associated with HH. At the present time, the CAP does not recommend the use of genetic tests for routine screening of asymptomatic persons (population screening).
Liver biopsy
Liver biopsy, used to assess iron deposition in hepatic cells, has long been used to establish a diagnosis of HH. In addition, liver biopsy will determine the presence of cirrhosis, fibrosis, and other abnormalities. Results of liver biopsy are also useful in ruling out other causes of iron overload. The necessity for performing a liver biopsy for HH diagnosis is controversial, although it is considered a safe and minor procedure by most hepatologists.30 As with other invasive procedures, there are inherent risks. The CAP recommends liver biopsy of patients thought to have hemochromatosis.8
Iron in the liver biopsy sample is assessed several ways. The hepatic iron index (HII) is calculated based on the concentration of iron (micromoles of iron per gram dry weight) in the tissue sample divided by the patient's age in years. Normal individuals have a HII of
Quantitative phlebotomy
An alternative to liver biopsy as a means of determining iron overload may be provided by quantitative phlebotomy, although this has not been well established. The removal of four to five grams of iron through documented successive phlebotomies (16 to 20 phlebotomies) without development of anemia is indicative of iron overload.7,8 Quantitative phlebotomy is useful in patients for whom liver biopsy is contraindicated or refused.
TREATMENT AND MANAGEMENT
Treatment of HH consists of two phases: initial and long-term maintenance.
Initial treatment
The initial or iron-depletion phase of treatment typically consists of removing one unit (450 mL) of whole blood once or twice weekly. Each unit of blood contains approximately 250 mg of iron. Prior to beginning phlebotomy, the hemoglobin and hematocrit must be checked. Treatment goals include inducing a mild anemia due to blood loss, without the development of debilitating symptoms of anemia. The number of phlebotomies needed to reduce iron levels and induce anemia is related to degree of initial iron overload. In one study, the mean rate of phlebotomy for iron depletion was 2.6 units per month.31
Patients are typically referred to a hematologist or gastroenterologist during the initial treatment phase. Most (76%) patients receive therapeutic phlebotomy services in a hospital or doctor's office. Patients may also undergo phlebotomy at a blood center. Although blood collected from persons with HH is not accepted for use as transfusion or blood products, 54% of HH patients have attempted to donate blood and were excluded, according to one survey. Persons undergoing therapeutic phlebotomy are charged for these services, and the majority of persons report they have full or partial insurance coverage.31
The initial phase continues until excess stored iron is removed and SF levels decrease to approximately 50 ng/mL.30 The CAP recommends continuing phlebotomy until SF levels reach 20 ng/mL.8 SF and hemoglobin levels should be periodically monitored during this phase. Removal of excess stored iron may take from one month to three years, although the mean duration has been shown to be 13 months.31
Treatment of HH patients with iron-chelating agents such as desferroxamine is usually not recommended due to the ease and efficacy of phlebotomy.8
Long-term maintenance
Long-term maintenance typically consists of removal of an average of two to six units of whole blood yearly. Monitoring of hemoglobin and SF levels determine the frequency of phlebotomy. SF levels should be maintained at concentrations of no more than 100 ng/mL. The primary care physician may manage patient care during long-term maintenance. For a comprehensive review of treatment protocols, the reader is referred to the CAP guidelines.8
Dietary recommendations
Compared to the importance of therapeutic phlebotomy in the management of iron levels in HH, dietary factors probably play a minor role.32 Some clinicians, however, recommend limiting iron ingestion through dietary restrictions to patients with HH.33 Limiting red meat consumption and avoiding use of cast iron cookware for food preparation may be advised. Because vitamin C enhances iron absorption, food and beverages high in vitamin C should be avoided with meats, but can be consumed between meals. Use of vitamin C supplements should be limited. Alcohol consumption should be limited to moderate amounts, and completely avoided by patients with liver disease. Shellfish, particularly oysters, should be thoroughly cooked to avoid infection with Vibrio vulnificus.32 A 50% mortality rate has been reported for patients with hepatic dysfunction who become infected with this organism.34 Tobacco is high in iron content and should be avoided.
Tea and dietary fiber may impede iron absorption and are sometimes recommended. There is some evidence that using antacids (calcium carbonate) may also impair iron absorption.13 Persons with HH should be cautioned to refrain from using over-the-counter medications containing iron, such as some vitamin supplements. Some patients may benefit from dietary counseling by a dietitian.
PROGNOSIS
The major determinant of prognosis is the degree of organ damage from iron overload at the point of diagnosis. The presence of cirrhosis reduces life expectancy.35 Hemochromatosis patients with cirrhosis at the time of diagnosis were over five times more likely to die compared to hemochromatosis patients without cirrhosis.36
Based on current data, at least 50% of males and 25% of females homozygous for the C282Y mutation will develop organ-damaging and life-threatening disease. Damage that has occurred to tissues and organs is irreversible, but further damage can be halted with treatment.
When there is no evidence of cirrhosis at time of diagnosis, life expectancy can be equal to that of persons without HH.35,36 With proper management of HH through phlebotomy, these affected individuals have good long-term outcomes.35
MORTALITY
Hepatocellular carcinoma associated with cirrhosis, hepatic failure and cardiac failure are the most common causes of death in persons with HH. Compared to the normal population, liver cancer is over 200 times more prevalent as a cause of death in persons with HH. Cardiomyopathy, diabetes, and cirrhosis are all more common causes of death among persons with HH than among normal persons.35 The earlier HH is detected, before the onset of severe organ damage, the lower the risk of mortality.
There is a large discrepancy between HH incidence as determined by laboratory test data and the frequency of reporting HH on death certificates." Underreporting on death certificates may be due to a variety of reasons, but failure to diagnose HH is certainly one possibility.
DIFFERENTIAL DIAGNOSIS
HH must be differentiated from causes of secondary iron overload caused by hematologic disorders or metabolic defects. Several types of anemia result in accumulation of excess iron, including the thalassemias, sideroblastic anemias, and congenital dyserythropoietic anemias. Liver disease, including alcoholic and nonalcoholic cirrhosis and viral hepatitis are also causes of iron overload and may result in increased TS levels.38 Other conditions associated with iron overload include rare defects of iron transport and porphyria cutanea tarda. Patients who undergo multiple transfusions and persons who overuse prescribed or over-the-counter iron supplements are also at risk for iron overload.30
Patient history, liver biopsy, and/or biochemical and hematologic analyses may be needed to establish a diagnosis of conditions causing secondary iron overload. DNA tests for common mutations are very likely the most important diagnostic tool for identifying HH as the cause of iron overload.29 In some patients, both secondary causes and HH may be contributing to iron overload.
IRON OVERLOAD IN AFRICAN AMERICANS
Iron overload is also observed in African Americans. An excess of hepatic iron was demonstrated in 1.5% of African Americans in an autopsy series, and a high incidence of hyperferritemia in African Americans was observed in a large nutritional study.39 However, the incidence of HH caused by the common genetic mutations in African Americans is estimated to be only 6% to 12% of that found in the Caucasian population.
African iron overload is a common problem in certain areas of sub-Saharan Africa, affecting over 10% in some groups. DNA analysis of African patients with iron overload indicates the possibility of an iron-loading gene, although the locus has not been identified. None of the patients in this study had the C282Y mutation. Iron overload in this population is thought to result from an interaction of a genetic defect with high consumption of iron-- laden beer.40
In a study of African Americans with iron overload, laboratory test results differed from those expected for patients with HH and more closely resembled the African form of iron overload. Mean TS and SI values were lower compared to those for Caucasians, and iron deposits within the liver demonstrated a different pattern from that seen in HH. Iron overload in subjects in this study could not be explained by excessive consumption of dietary iron. The authors concluded that African Americans with iron overload may have a heritable disorder different from that of HH.39
It is possible that African Americans with iron overload do not have the common mutations of C282Y or H63D, but may have a different as yet unidentified genetic defect. More studies are needed in this population.
HEMOCHROMATOSIS IN WOMEN
In spite of HH having an autosomal recessive inheritance pattern, all studies of patients with HH to date include more male than female subjects.8 Similarly, population screening has resulted in the identification of more cases of HH in men than in women, although women have been excluded from some studies.41 Because of iron loss from menstruation and childbearing, clinical expression of HH has traditionally been thought to occur later in life (after menopause) and to be less severe in women than in men. However, women do develop organ damage and die from HH. It is possible that HH is sometimes overlooked as a potential diagnosis in symptomatic female patients, leading to a bias in reporting of incidence and unnecessary morbidity and mortality.
Recent data has shown that women can have full phenotypic expression of HH, including cirrhosis. Compared to men, women have a lower incidence of cirrhosis and diabetes, but a higher incidence of fatigue and skin pigmentation at time of presentation. While symptoms at presentation differ, age at presentation is similar for women and men. Pre-menopausal women with severe HH were identified in this study. Women with HH have lower SF levels than men, but comparable amounts of hepatic iron. The authors conclude that HH is only slightly underexpressed in women and that presenting symptoms are different.42
Women should be included in population screening for HH, but investigators should take into account that lower iron levels may be present in women with HH than in men. Appropriate reference ranges should be consulted, as those for SF are lower for women than for men. Because at least 30% of women with HH who are less than age 30 may have TS levels in the normal range, tests should be repeated later in women in whom there is a possibility of HH, e.g., relatives of affected individuals.2 Finally, physicians need to consider HH as a diagnosis in all women who present with unexplained fatigue, arthralgia, or pigmentation 42
HEMOCHROMATOSIS IN CHILDREN
Although commonly considered to cause disease only in adults, HH can also be found in children, sometimes as young as two years of age. HH is the second most common cause of iron overload in children, after that due to transfusion. Younger persons with HH tend to demonstrate cardiac and gonadal problems, rather than the cirrhosis and diabetes seen in adult patients. Young patients with cardiomyopathies, hypogonadism, amenorrhea, diabetes, and arthritis, or those whose parents have HH, should be tested for HH. Iron overload may become severe by the second to third decade of life.14
A form of juvenile hemochromatosis (JH) has been described in recent studies of affected Italian and French families. Members of these families lack the common HFE gene mutations. These studies suggest a linkage of JH to a locus on the long arm of chromosome 1.43,44 Other studies in the United Kingdom have found affected children to be heterozygous for the more common C282Y mutation, and suggest that JH is a genetically heterogeneous disorder distinct from the more common adult type.45
There is also a rare and usually fatal neonatal form of iron overload. The basis for this severe intrauterine accumulation of iron remains unknown but appears not to be caused by common HFE mutations.38
UNIVERSAL SCREENING
Hemochromatosis is an excellent candidate for universal screening due to its prevalence, associated morbidity and mortality, benefits of early diagnosis, and ease of effective treatment. A goal of population screening is to identify persons with HH before the symptoms of iron overload become clinically evident. Phlebotomy treatment may then be initiated prior to the occurrence of irreversible organ damage. Screening can be performed using simple and relatively low-cost laboratory analyses that evaluate phenotypic expression of HH.46 A screening study conducted by the Centers for Disease Control and Prevention (CDC) estimated that use of TS would identify an estimated 1.4 to 2.5 million adults in the US with elevated results.47
Current recommendations by the CAP include using iron studies, rather than DNA tests to screen for HH.8 TS is a good screening test, as its elevation is the first phenotypic expression of excess iron transport from the intestine. Increased TS should occur before significant iron over-loading occurs. SF levels reflect total-body iron stores and increase as iron accumulates in tissue.7 A combination of TS, followed by SF will identify most homozygotes in a population of healthy individuals. Among presumably healthy blood donors in Utah, a TS of 62% or greater was useful in identifying homozygotes.2 Screening criteria differ in other studies. In an Australian study, a TS threshold of 45% was found to identify affected persons with few false positives.27
The optimal age at which to screen in a cost-effective manner has yet to be determined, as iron overload in persons less than 20 is rare.41 Screening younger persons may be ineffective if iron levels have not increased to levels considered significant by screening criteria. CAP guidelines recommend screening persons over the age of 20.(8)
Universal screening of asymptomatic individuals is thought to be more cost-effective than treating affected persons once symptoms of HH develop.48,49,50 Studies in Canada suggest that screening of healthy blood donors for HH has the potential to improve societal health status while decreasing long-term healthcare costs.51 Routine screening of Caucasian men over age 30 is recommended based on results of a study in California.41 Workplace screening of employees of one North Carolina company was concluded to be cost effective and to compare favorably to other common workplace screening programs, e.g., mammography.52
Neonatal screening has been conducted in a limited study in Australia. Specimens were taken from dried blood spots collected on cards and tested for C282Y using PCR amplification. In this sample of 1,660 mostly Caucasian neonates, 5% were homozygous for the mutation.53 It is conceivable that genetic screening could be incorporated into existing neonatal screening programs conducted by most state health departments. In North Carolina, neonatal screening using dried blood spot samples is performed for sickle cell disease, phenylketonuria, primary hypothyroidism, galactosemia, and congenital adrenal hyperplasia. All of these disorders are less common than HH. Before universal screening is instituted, it will be advisable to learn more about disease expression, relationships between genotype and phenotype, optimal screening criteria, cost-effectiveness of screening programs, and social issues. The Working Group on Research Priorities, a panel composed of clinicians, basic scientists, and experts in public health and laboratory medicine, has identified several important aspects of HH requiring firther study. The panel's most important goal is characterization of the relationship between genotype and phenotype. Other objectives include development of an optimal approach to screening, cost-effectiveness analysis, and determination of ethical, legal, and social implications. Multi-center research studies to address these priorities have been recommended.54
ETHICAL CONSIDERATIONS
Because HH is an inherited disorder, care must be taken to minimize the potential for genetic discrimination. As with other genetic disorders, health or life insurance could be cancelled or difficult to obtain if a diagnosis is made. Persons with asymptomatic hemochromatosis have reported instances of genetic discrimination affecting employment and insurance.55
Particularly troubling is the issue of variable phenotypic expression of the C282Y and H63D mutations. If DNA tests are used for screening, the potential exists for a genetic diagnosis only and possible discrimination in a person who will never develop clinical iron overload.
Genetic counseling may be advisable for persons diagnosed with HH, for family members, and for persons considering being tested as part of screening programs. The health benefits associated with early detection through screening must be weighed against the potential for harm due to genetic discrimination.
USE OF BLOOD FROM PHLEBOTOMIES
Blood units collected therapeutically from persons with HH do not meet acceptability criteria established by the US Food and Drug Administration for use in transfusion. To address safety issues, only blood collected from volunteer donations is presently acceptable for transfusion.24 Because HH patients may derive personal benefits from phlebotomies, they are not considered to be strictly volunteers, and their blood is not acceptable for transfusion. There is no evidence, however, that a genetic disease such as HH can be transmitted through blood transfusion.
Blood from persons with HH has been used in Canada, Australia, Norway, Sweden, and South Africa." Recent studies have estimated the positive impact of potentially using blood from therapeutic phlebotomies of persons with HH on the US blood supply. Although they may have somewhat lower eligibility rates because of other factors, many persons with HH would be acceptable donors.57 It is possible that using blood drawn from persons with HH would increase the annual US blood supply by up to 3 million units 56
FUTURE DIRECTIONS
The need for a variety of initiatives dealing with HH was discussed at a meeting sponsored by the CDC in 1997, entitled Iron Overload, Public Health, and Genetics: Challenges and Opportunities. A unified system of public health surveillance for HH is needed to acquire better information about the natural history of the disorder, incidence, relationships of genotype to phenotype, best methods of detection, and treatment. Consensus on case definitions of HH remains to be established and must be addressed.58 Public and healthcare provider education is another targeted need. In order for HH to be detected early, physicians must be better informed about the actual incidence of the disorder, basic concepts of genetics, the value of TS as a screening test, and recommended diagnostic algorithms. Continuing medical education programs may be valuable in addressing these needs.59,60 Other strategies to increase detection of HH include identifying local physician-leaders and improving education in medical schools. The public, including policy-makers and payers must be educated about the benefits of early detection of HH.61
CONTINUATION AND DISCUSSION OF CASE STUDY
As a result of finding elevated TS and SF levels, the patient's physician ordered DNA tests for the common mutations associated with HH. She was found to be homozygous for the C282Y mutation and was referred to a gastroenterologist for evaluation of liver disease. The patient declined liver biopsy. She began weekly phlebotomy therapy at a local hospital, and within 50 weeks her SF had dropped to 175 mg/L. Liver enzymes have also decreased to within reference ranges. She still reports minor joint pain. She remains under the care of her primary care physician and at present undergoes bi-monthly phlebotomies.
The patient reports that she is fatigued for 24 to 48 hours following blood collection, resulting in occasional absences from work. Also, phlebotomists sometimes have difficulty with the venipuncture procedure, missing the vein, or collecting the entire unit. Although her health insurance plan pays for the phlebotomy treatments, minus a small co-payment, the patient wishes her blood could be "donated" and used for treatment of patients in need of transfusion.
The patient was referred to a genetic counselor, and as a result, the patient's siblings and mother were also referred for genetic testing. Her sister was found to also be homozygous for the C282Y mutation. The patient's mother was found to be heterozygous for C282Y Her brother has stated he does not want to be tested. The patient's adult children were advised to undergo either biochemical screening tests for iron overload or genetic testing, as their father has not been tested for HH.
This case illustrates several important aspects of HH. HH is prevalent among Caucasians, especially those of northern European descent, and is found in both sexes. In this case, diagnosis of the patient's disorder led to detection of HH in another family member. It is possible that the patient's father also had undiagnosed HH, leading to his liver disease and death at age 70.
Symptoms of HH are nonspecific and often become apparent only in middle age. Upon finding hepatomegaly and joint inflammation in this patient, the physician was prompted to order iron studies in addition to other screening tests. The patient was fortunate that her physician suspected HH as a possible diagnosis so that treatment could begin before her condition worsened and organ damage became irreversible. Symptoms in women appear somewhat later than in men, possibly due to the protective effects offered by iron loss through menstruation and pregnancy.
Phlebotomy offers a safe, effective, and relatively low cost treatment for HH. This patient reports experiencing some of the negative aspects of treatment which have been reported by other patients.62 She is fortunate to be covered by medical insurance, as healthcare institutions typically charge for therapeutic phlebotomy. The desire that her blood be used for transfusion rather than discarded is shared by other HH patients. Phlebotomy therapy has been effective in reducing the patient's iron overload and resolution of some symptoms has occurred.
The role of genetic testing in hemochromatosis is not without controversy. In the case of this patient, genetic testing was valuable in establishing a diagnosis of HH following detection of elevated iron levels. DNA tests were also useful in determining the presence of HH in the patient's sister. That her brother declined testing illustrates the reservation that some individuals express concerning possessing genetic information. Because of the potential for discrimination in future employment and obtaining health insurance, it might be advisable to postpone genetic testing of the patient's minor child until she/he is older.
REFERENCES
1. Feder JN, Gnirke A, Tsuchihashi Z, and others. A novel MCH Class I-like gene is mutated in hereditary hemochromatosis. Nat Genet 1996;13:339-409.
2. Edwards CQ, Griffen LM, Goldgar D, and others. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Engl J Med 1988:318:1355-62.
3. Phatak PD, Sham RL, Raubertas RF and others. Prevalence of hereditary hemochromatosis in 16,031 primary care patients. Ann Intern Med 1998;129:954-61.
4. Burke W, Thomson E, Khoury MJ, and others. Hereditary hemochromatosis: Gene discovery and its implications for population-based screening. JAMA 1998;280:172-8.
5. McLaren CE, Gordeuk VR, Looker AC, and others. Prevalence of heterozygotes for hemochromatosis in the Caucasian population of the United States. Blood 1995:86:2021-7.
6. Merryweather-Clarke AT, Pointon JJ, Shearman JD, and others. Global prevalence of putative haemochromatosis mutations. J Med Gen 1997;34:275-8. 7. Powell LW, George DK, McDonnell SM, and others. Diagnosis of hemochromatosis. Ann Intern Med 1998;129:925-31.
8. Witte DL, Crosby WH, Edwards CQ, and others. Hereditary Hemochromatosis. Clin Chim Acta 1996;245:139-200.
9. Fellitti V, Perlman M, Howard N. Iron overload disorders among Hispanics-San Diego, California, 1995. MMWR 1996:45:991-3.
10. McCord JM. Iron, free radicals, and oxidative injury. Seminars in Hematology 1998;35:5.
11. McKenzie SB. Textbook of hematology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1996.
12. Andrews NC, Levy JE. Iron is hot: an update on the pathophysiology of hemochromatosis. Blood 1998;92:1845-51.
13. Huebers HA, Finch CA. Transferrin: physiologic behavior and clinical implications. Blood 1984;64:763-7.
14. Haddy TB, Castro OL, Rana SR Hereditary hemochromatosis in children, adolescents, and young adults. Am J Pediatr Hematol Oncol 1988; 10:23-4.
15. Press RD. Hereditary hemochromatosis. Arch Pathol Lab Med 1999;123:1053-9.
16. Feder JN, Penny DM, Irrinki A, and others. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Nati Acad Sci 1998;95:1472-7.
17. Waheed A, Parkkila S, Zhou XV, and others. Hereditary hemochromatosis: Effects of C282Y and H63D mutations on association with b-2 microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Nad Acad Sci 1997;94:12384-9.
18. Beutler E, Gelbart T, West C, and others. Mutation analysis in hereditary hemochromatosis. Blood Cells Mol Dis 1996;22:187-94.
19. Joua-nolle AM, Gandon G, Jesequel PI and others. Haemochromatosis and HLA-H. Nat Genet 1996;14;251-2.
20. Jazwinska EC, Cullen LM, Busfield F, and others. Haemochromatosis and HLA-H. Nat Genet 1996;14;249-51.
21. Ajioka RS, Jorde LB, Gruen JR, and others. Haplotype analysis of hemochromatosis: evaluation of different linkage-disequilibrium approaches and evolution of disease chromosomes. Am J Hum Genet 1997;60:1439-47.
22. Ryan E, O'keane C, Crowe J. Hemochromatosis in Ireland and HFE. Blood Cells Mol Dis 1998:24;428-32.
23. Lucotte G. Celtic origin of the C282Y mutation in hemochromatosis. Blood cells Mol Dis 1998:24;433-8.
24. Sacher RA Hemochromatosis and blood donors: a perspective. Transfusion 1999;39:551-4.
25. Bothwell TH, MacPhail AP. Hereditary hemochromatosis: etiologic, pathologic, and clinical aspects. Semin Hematol 1998;35:55-71.
26. Determination of Serum Iron, Total Iron-Binding Capacity and Percent Transferrin Saturation; Approved Standard H 17-A. National Committee for Clinical Laboratory Standards, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 1987-1989, USA, 1998.
27. McLaren CE, McLachlan GJ, Halliday JW, and others. Distribution of transferrin saturation in an Australian population: relevance to the early diagnosis of hemochromatosis. Gastroenterology 1998; 114:543-9.
28. Burns, Carl A; Ashwood Edward R, editors. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia: WB Saunders Co; 1999.
29. Press RD, Flora K, Gross C, and others. Hepatic iron overload: direct HFE (HLA-H) mutation analysis vs quantitative iron assays for the diagnosis of hereditary hemochromatosis. Am J Clin Path 1998:109:577-84.
30. Bacon BR Diagnosis and management of hemochromatosis. Gastroenterology 1997;113:995-9.
31. McDonnell SM, Grindon AJ, Preston BL, and others. A survey of phlebotomy among persons with hemochromatosis. Transfusion 1999;39:651-6.
32. Barton JC, McDonnell SM, Adams PC, and others. Management of hemochromatosis. Ann Intern Med 1998;129:932-9.
33. Roeckel IE, Dickson LG. Understanding iron absorption and metabolism, aided by studies of hemochromatosis. Ann Clin & Lab Sci 1998;28:30-3.
34. Baron EJ, Peterson LR, Finegold SM. Diagnostic microbiology, 9th ed. St. Louis: Mosby; 1994. p 430-2.
35. Niederau C, Fischer R, Sonnenberg A, and others. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med 1985:313:1256-63.
36. Adams PC, Speechley M, Kertesz AE. Long-term survival analysis in hereditary hemochromatosis. Gastroenterology 1991; 101:368-72.
37. Yang Q, McDonnell SM, Khoury MJ, and others. Hemochromatosis-associated mortality in the United States from 1979 to 1992: an analysis of multiple-cause mortality data. Ann Intern Med 1998; 129:946-53.
38. Bottomly SS. Secondary iron overload disorders. Semin Hematol 1998;35:77-86.
39. Barton JC, Edwards CQ, Bertoli LF, and others. Iron overload in African Americans. Am J Med 1995;99:616-23.
40. McNamara L, MacPhail AP, Gordeuk VR, and others. Is there a link between African iron overload and the described mutations of the hereditary haemochromatosis gene? Br J Haematology 1998:102;1176-8.
41. Baer DM, Simons JL, Staples RL, and others. Hemochromatosis screening in asymptomatic ambulatory men 30 years of age and older. Am J Med 1995;98:464-8.
42. Moirand R, Adams PC, Bicheler V, and others. Clinical features of genetic hemochromatosis in women compared with men. Ann Intern Med 1997;123:105-10.
43. Cazzola M, Cerani P, Rovati A, and others. Juvenile genetic hemochromatosis is clinically and genetically distinct from the classical HLA-related disorder (letter). Blood 1998;92:2979-81.
44. Pinson S, Yaouanq J, Jouanolle AM, and others. Non-C282Y familial iron overload: evidence for locus heterogeneity in haemochromatosis. J Med Genetics 1998;35:954-6.
45. Kelly AL, Rhodes DA, Roland JM, and others. Hereditary juvenile haemochromatosis: A genetically heterogeneous life-threatening iron-storage disease. QJM 1998:91;607-18.
46. Edwards CQ Kushner JP Screening for hemochromatosis. N Engl J Med 1993:328:1616-20.
47. Looker AC, Johnson CL. Prevalence of elevated serum transferrin saturation in adults in the United States. Ann Intern Med 1998;129:940-5.
48. Balan V, Baldus W, Fairbanks V, and others. Screening for hemochromatosis: a cost-effectiveness study based on 12,258 patients. Gastroenterology 1994;107:453-9.
49. Buffone GJ, Beck JR. Cost-effectiveness analysis for evaluation of screening programs: Hereditary hemochromatosis. Clin Chem 1994;40:1631-6.
50. Phatak PD, Guzman G, Woll JE, and others. Cost-effectiveness of screening for hereditary hemochromatosis. Arch Intern Med 1994;154:769-76.
51. Adams PC, Gregor JC, Kertesz AE, and others. Screening blood donors for hereditary hemochromatosis: Decision analysis model based on a 30-year database. Gastroenterology 1995;109:177-88.
52. Stave GM, Mignogna JJ, Powell GS, and others. Evaluation of a workplace hemochromatosis screening program. Am J Prev Med 1999:16:303-6.
53. Cullen LM, Summerville L, Glassick TV, and others. Neonatal screening for the hemochromatosis defect. Letter to editor. Blood 1997;90:4236-7.
54. Brittenham GM, Franks AL, Rickles FR. Research priorities in hereditary hemochromatosis. Ann Intern Med 1998;129:993-6.
55. Alper JS, Geller LN, Barash CI, and others. Genetic discrimination and screening for hemochromatosis. J Public Health 1994; 15:345-58.
56. Jeffrey G, Adams PC. Blood from patients with hereditary hemochromatosis-a wasted resource? Transfusion 1999;39:549-54.
57. Barton JC, Grindon AJ, Barton NH, and others. Hemochromatosis probands as blood donors. Transfusion 1999;39:578-85.
58. Wetterhall SF, Cogswell ME, Kowdley KV. Public health surveillance for hereditary hemochromatosis. Ann Intern Med 1998;129:980-6.
59. Franks AL, Marks JS. Introduction to supplement on iron overload, public health, and genetics. Ann Intern Med 1998;129:923-4.
60. McDonnell SM, Phatak PD, Felitti V, and others. Screening for hemochromatosis in primary care settings. Ann Intern Med 1998; 129:962-70.
61. McDonnell SM, Witte DL, Cogswell ME, and others. Strategies to increase detection of hemochromatosis. Ann Intern Med 1998;129:987-92.
62. Seamark CJ, Hutchinson M. Should asymptomatic haemochromatosis be treated? BMJ 2000;320:1314-6.
Rebecca J Laudicina PhD CLS(NCA) is Associate Professor Clinical Laboratory Science, Principal Investigator, Hemochromatosis Education and Screening Project at The University ofNorth Carolina at Chapel Hill NC.
Vicky A LeGrys DA CLS(NCA) is Professor, Clinical Laboratory Science, The University ofNorth Carolina at Chapel Hill NC.
Addressfor correspondence: Dr Rebecca Laudicina, Medical School Wing E, CB#7145, UNC-Chapel Hill, Chapel Hill, NC 27599-- 7145. (919) 966-3011, (919) 966-8384 (fax). Rebecca_Laudicina@med.unc.edu
Rebecca Laudicina is the Focus: Iron Overload guest editor.
Copyright American Society for Clinical Laboratory Science Summer 2001
Provided by ProQuest Information and Learning Company. All rights Reserved