Hivid
Zalcitabine (2'-3'-dideoxycytidine, ddC), also called dideoxycytidine, is a nucleoside analog reverse transcriptase inhibitor (NARTI) sold under the trade name Hivid®. more...
Zalcitabine is the least potent of all antiretroviral drugs, is inconvenient to take, and has serious side effects. For these reasons it is now rarely used to treat human immunodeficiency virus (HIV).
History
Zalcitabine was developed in the National Cancer Institute (NCI) by Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan at the National Cancer Institute (NCI). Like didanosine, it was then licensed because the NCI may not market drugs. The National Institutes of Health (HIH) thus licensed it to Hoffman LaRoche Co.
Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV infection and AIDS. It was approved on Jun 19, 1992 as a monotherapy and again in 1996 for use in combination with Zidovudine (AZT). Using combinations of NRTIs was in practice prior to the second FDA approval and the triple drug combinations with dual NRTIs and a protease inhibitor (PI) were not far off by this time.
Mechanism of action
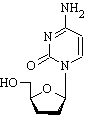
Zalcitabine is an analog of pyrimidine. It is a derivative of the naturally existing deoxycytidine, made by replacing the hydroxyl group in position 3' with a hydrogen.
It is phosphorylated in the T cell and other HIV target cells into its active triphosphate form, ddCTP. This active metabolite works as a substrate for HIV reverse transcriptase, and also by incorporation into the viral DNA, hence terminating the chain elongation due to the missing hydroxyl group. Since zalcitabine is a reverse transcriptase inhibitor it possess activity only against retroviruses. It is used for the treatment of HIV infection only in combination with zidovudine.
Zalcitabine has a very high oral absorption rate, of over 80%. The most common side effect in the beginning of the treatment is nausea and headache. The more serious side effects are peripheral neuropathy, oesophagitis and pancreatitis.
Read more at Wikipedia.org