METHOD OF PREPARATION
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the isosorbide dinitrate with the propylene glycol and sufficient 70% alcohol to volume and mix well.
4. Package in a spray container and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed.
STABILITY
A beyond-use date of up to 6 months can be used for this preparation.1
USE
Isosorbide dinitrate topical spray has been used to increase peripheral circulation. The concentration of this preparation can be easily adjusted to the needs of the patient.
QUALITY CONTROL
Quality-control assessment can include weight/volume, specific gravity, active-drug assay, color, rheological properties/pourability, physical observation and physical stability (discoloration, foreign materials, gas formation, mold growth).2
DISCUSSION
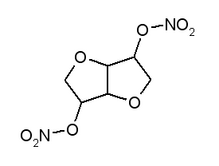
Isosorbide dinitrate (C^sub 6^H^sub 8^N^sub 2^O^sub 8^, MW 236.14) occurs as an ivory-white, odorless powder in diluted form. Undiluted isosorbide dinitrate is very slightly soluble in water and sparingly soluble in alcohol. Isosorbide Concentrate USP is an aqueous solution containing not less than 70.0 g and not more than 80.0 g of C^sub 6^H^sub 10^O^sub 4^ in each 100 g. Diluted isosorbide dinitrate is a dry mixture of isosorbide dinitrate with lactose, mannitol or suitable inert excipients to permit safe handling. It may contain up to 1.0% of a suitable stabilizer such as ammonium phosphate. It carries the caution: "Caution-Exercise proper precautions in handling undiluted isosorbide dinitrate, which is a powerful explosive and can be exploded by percussion or excessive heat. Only exceedingly small amounts should be isolated." It is stored in tight containers.1
Propylene glycol (C^sub 3^H^sub 8^O^sub 2^, MW 76.09) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. It has a specific gravity of 1.038 g/mL and is miscible with acetone, chloroform, 95% ethanol, glycerin and water. It is not miscible with fixed oils or light mineral oil; it will, however, dissolve some essential oils. A 2% v/v aqueous solution of propylene glycol will be iso-osmotic with serum. Propylene glycol is used as a humectant in topicals (~15% concentration), a preservative in solutions and semisolids (15% to 30% concentration), and a solvent or cosolvent in aerosols (10% to 30% concentration), oral solutions (10% to 25% concentration), patenterais (10% to 60% concentration) and topicals (5% to 80% concentration). Propylene glycol is actually a better solvent than glycerin. It is similar to ethanol as an antiseptic and is also used in cosmetics and in the food industry as a vehicle for flavors and as a vehicle for emulsifiers. It is stable and may be mixed with numerous other solvents. Aqueous solutions of propylene glycol can be sterilized by autoclaving. Since propylene glycol is hygroscopic, it should be stored in an airtight container and protected from light. Incompatibilities include potassium permanganate.3
Alcohol (C^sub 2^H^sub 5^OH, MW 46.07, ethyl alcohol, ethanol, grain alcohol) is a clear, colorless mobile and volatile liquid with a slight, characteristic odor and a burning taste. It is used as an antimicrobial preservative (>10% concentration), a disinfectant (60% to 90% concentration), a solvent in injectable and oral liquids (variable concentration), and a solvent in topical products (60% to 90% concentration). Alcohol USP refers to 95% ethanol and dehydrated alcohol refers to 99.5% alcohol. Its specific gravity is between 0.812 and 0.816, and its boiling point is 78.15°C. It is miscible with chloroform, glycerin and water; and its solutions may be sterilized by autoclaving or by filtration. It should be stored in a cool place. It is incompatible with oxidizing materials in acidic conditions. With alkalies, it may darken in color. When added to aqueous solutions of organic salts or acacia, they may precipitate. Alcohol is incompatible with aluminum containers; it may interact with some drugs.4
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 1044-1046, 2345-2349, 2767.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC1999; 3(2): 146-147.
3. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 521-523.
4. Owen SC. Alcohol. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 13-15.
Copyright International Journal of Pharmaceutical Compounding Mar/Apr 2005
Provided by ProQuest Information and Learning Company. All rights Reserved