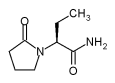
UCB Pharma, Inc. has been granted a priority
review for the supplemental new drug application (sNDA) seeking approval of
its leading anti-epilepsy drug (AED) Keppra®* (levetiracetam) as add-on
therapy in children and adolescents with partial seizures in the USA.
Under a priority review, the Food and Drug Administration (FDA) sets a
six-month target for deciding whether to approve a new drug application,
instead of the standard target of 10 months after the date the application
is filed. A priority designation is intended for products that address
unmet medical needs and, if approved, would be a significant improvement on
products already on the market.
UCB Pharma, Inc. submitted the pediatric sNDA for Keppra® on 20 December
2004, requesting approval of Keppra® for the adjunctive treatment of
partial seizures in children down to four years of age. Keppra® was
first marketed in the year 2000 and is now the most prescribed second
generation AED for adults with partial onset seizures in the USA.(1) The
introduction of Keppra for children, as early as Q3 2005 in the U.S. and
EU, will give even more patients the opportunity to receive this innovative
medicine.
The application is based on recent pivotal trial results in 198 patients
showing excellent efficacy and safety in children aged 4-16 years with
refractory epilepsy.(2) The children who took part in the study were taking
one or two other AEDs at entry.(2) Seven percent of children who took
Keppra® became seizure free during the 14 week double-blind,
placebo-controlled treatment period, compared with 1% of those taking
placebo. Responder rates -- a 50% or greater reduction in seizures -- were
45% on Keppra® treatment and 20% on placebo (p=0.0002).(2)
Dr. Tracy Glauser, Director Comprehensive Epilepsy Program, Cincinnati
Children's Hospital, and principal investigator of the study, stressed the
importance of early, aggressive treatment of childhood seizures to lesson
the risk of injury to the child, maximize school performance, and thereby
improve their quality of life.
"Keppra® was effective and well tolerated by the children in our study,
many of whom had been on eight or nine different drugs before trying
Keppra®," he said.
About Keppra®
In the U.S., Keppra® is approved for adjunctive therapy in the treatment
of partial onset seizures in adults with epilepsy. Keppra® is available
in 250, 500 and 750 mg tablets and a grape-flavored (100 mg/mL) oral
solution for patients who prefer a solution or have difficulty swallowing
tablets. Taken with or without food, the effective recommended starting
dose of Keppra® is 1,000 mg/day given twice daily (500 mg bid). Since
its launch, Keppra® has had more than 500,000 unique patient starts in
the United States.(3)
Keppra® use is associated with the occurrence of central nervous system
adverse events, including somnolence and fatigue, coordination
difficulties, and behavioral abnormalities as well as hematological
abnormalities. Keppra® dosing must be individualized according to renal
function status. In well-controlled clinical studies, the most frequently
reported adverse events associated with the use of Keppra® in combination
with other AEDs, not seen at an equivalent frequency among placebo-treated
patients, were somnolence, asthenia, infection and dizziness.
The recent identification of SV2A as a binding site for levetiracetam
confirms that Keppra® possesses a mechanism of action that is truly
distinct from that of all other AEDs.
*For full U.S. prescribing information consult www.keppra.com. Outside of
the U.S., please consult local prescribing information.
* Keppra® is a registered trademark of the UCB Group
About UCB Pharma
UCB Pharma, Inc., with U.S. headquarters in Smyrna, Georgia, is the North
American subsidiary of the global research-based pharmaceutical sector of
UCB S.A. UCB Pharma is a biopharmaceutical leader, specializing in the
fields of central nervous system disorders, allergy and respiratory
disease, immune and inflammatory disorders and oncology. UCB Pharma's key
products are Keppra® (antiepileptic), Zyrtec®† (antihistamine), and
Tussionex® (antitussive). UCB Pharma employs over 8,000 people operating
in over 100 countries, and in 2003 achieved sales of EUR 1.5 billion.
Worldwide headquarters are located in Brussels, Belgium.
†Zyrtec® is licensed to, and co-promoted with, Pfizer in the U.S.
References
(1) Available from: IMS Health, National Disease and Therapeutic Index(TM).
Accessed Oct 2004; based on an intent-to-treat (ITT) analysis; when
generalized seizures were specified, the data were excluded from this
analysis.
(2) Glauser TA, Gauer LJ, Chen L and LEV N159 Pediatric Study Group.
Multicenter, double-blind, placebo-controlled trial of adjunctive
levetiracetam (Keppra®) therapy (up to 60 mg/kg/day) in pediatric
patients with refractory partial epilepsy. Epilepsia 2004; 45 (supplement
7): 186 (B.03)
(3) Available from: Verispan Retail Pharmacy Database. Accessed May 2004.
Contact:
Lisa Garman
UCB Pharma, Inc.
Cell (404) 291-4772
lisa.garman@ucb-group.com