METHOD OF PREPARATION
Note: The lecithin:isopropyl palmitate solution can be prepared by mixing 0.2 g sorbic acid, 50 g of soy lecithin and 50 g of isopropyl palmitate. The Pluronic F127 solution can be prepared by mixing 0.2 g sorbic acid, 30 g of Pluronic F127 and sufficient purified water to make 100 mL.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Mix the ketamine hydrochloride and the ketoprofen with the propylene glycol to form a smooth paste.
4. Incorporate this paste into the lecithin:isopropyl palmitate solution and mix well.
5. Add sufficient Pluronic F127 20% gel to volume and mix using a shear-mixing technique until thoroughly mixed.
6. Package and label.
PACKAGING
Package in tight, light-resistant containers.1
LABELING
Keep out of reach of children. Use only as directed. For external use only.
STABILITY
A beyond-use date of up to 30 days can be used for this preparation.1
USE
Ketamine and ketoprofen Pluronic lecithin organogel have been used in the treatment of mild-to-moderate musculoskeletal pain.
QUALITY CONTROL
Quality-control assessment can include theoretical weight compared to actual weight, pH, specific gravity, active drug assay, color, clarity, texture-surface, texture-spatula spread, appearance, feel, rheological properties and physical observations.2
DISCUSSION
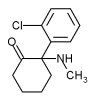
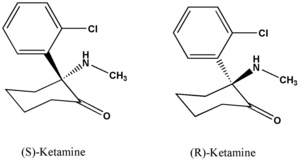
Ketamine hydrochloride (C^sub 13^H^sub 16^CINO.HCl, MW 274.2) is used as an anesthetic and analgesic. It occurs as a white crystalline powder with a slight characteristic odor. Approximately 1.15 mg is equivalent to 1 mg of ketamine base. It is soluble 1 g in 4 mL of water, in 14 mL of alcohol and in 60 mL of absolute alcohol.1,3
Ketoprofen (C^sub 16^H^sub 14^O^sub 3^, MW 254.28) occurs as a white or almost-white, odorless or almost-odorless crystalline powder. It is practically insoluble in water but freely soluble in alcohol and ether. It has a melting range between 92.0°C and 97.0°C. Ketoprofen has analgesic, anti-inflammatory and antipyretic properties and is an inhibitor of cyclo-oxygcnase.1,3
Propylene glycol (C^sub 3^H^sub 8^O^sub 2^, MW 76.09) occurs as a clear, colorless, viscous, practically odorless liquid with a sweet taste, somewhat resembling glycerin. It is miscible with acetone, 95% ethanol, glycerin and water.4
Lecithin (egg lecithin, soybean lecithin, vegetable lecithin) is a complex mixture of acetone-insoluble phosphatides; the composition and physical properties vary depending upon the source and the degree of purification. Physically, lecithin ranges from a viscous semiliquid to a powder. It is practically insoluble in water; when mixed with water, though, it hydrates to form emulsions. It should be stored in well-closed containers and be protected from light.5
Isopropyl palmitate (C^sub 19^H^sub 38^O^sub 2^, MW 298.51) is a colorless, mobile liquid with a very slight odor that is used as an emollient, oleaginous vehicle and a solvent. It is soluble in acetone, castor oil, cottonseed oil, alcohol and mineral oil. It is insoluble in water, glycerin and propylene glycol.6
Pluronic F127, a poloxamer, is a series of closely related block copolymers of ethylene oxide and propylene oxide. Poloxamers are available in different grades, either liquids or solids with average molecular weights ranging from 2,090 to 14,600. Poloxamers generally are white-colored, waxy, free-flowing granules or cast solids that are practically odorless and tasteless. Poloxamer 407 (Pluronic F-127) is generally available in powdered form. It is either odorless or may have a very mild odor. It is freely soluble in water, alcohol and isopropyl alcohol.7
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 27-National Formulary 22. Rockville, MD: US Pharmacopeial Convention, Inc.; 2004: 978, 1054-1056, 2345-2349, 2768.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC 1998; 2: 308-309.
3. Sweetman SC, ed. MARTINDALE: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002: 210-222, 1262-1263.
4. Weller PJ. Propylene glycol. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 521-523.
5. Fowler K. Lecithin. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 340-342.
6. Taylor AK. Isopropyl palmitate. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 314-315.
7. Collett JH. Poloxamer. In: Rowe RC, Sheskey PJ, Weller PJ, eds. Handbook of Pharmaceutical Excipients. 4th ed. Washington, DC: American Pharmaceutical Association; 2003: 447-450.
Copyright International Journal of Pharmaceutical Compounding Sep/Oct 2004
Provided by ProQuest Information and Learning Company. All rights Reserved