INTRODUCTION
Cocaine use continues to be a major public health problem. In 1994, according to the National Household Survey on Drug Abuse, an estimated 1.4 million Americans were current cocaine users; that is, they had used cocaine at least once in the past month (1). The rate of current cocaine use was highest among young adults, with approximately 60% of current cocaine users aged between 18 and 34. In spite of the magnitude of this problem, there are no proven pharmacological treatments for cocaine abuse. A pharmacological treatment theoretically could reduce cocaine use by blocking reinforcing properties of cocaine, reducing craving, increasing the aversive effects of cocaine, or substituting for cocaine effects. Based on these considerations, various agents acting on different receptor systems have been tested as treatment for cocaine abuse. These include antidepressants like desipramine (2, 3) and fluoxetine (4-6); dopamine agonists like bromocriptine (7), amantadine (8, 9), and bupropion (10); dopamine antagonists like haloperidol (11); and various other agents, like mazindol (12, 13), carbamazepine (14-17), naltrexone ( 18, 19), and disulfiram (20). None of these treatment modalities has shown consistently promising results.
The need to develop a medication that is consistently effective for cocaine abuse has brought attention to another class of agents called excitatory amino acid (EAA) antagonists. Most of the studies regarding the possible role of EAA antagonists for treatment of cocaine-related disorders have been conducted in animals. In rats, these agents have been shown to alter cocaine self-administration (21), reduce the locomotor stimulatory effects of cocaine (22), attenuate the neurochemical sequelae of cocaine (23, 24), and reduce the frequency of cocaine-induced seizures (25, 26).
Some laboratory studies have also shown an association between glutamate, the principal EAA, and release of dopamine. It has been demonstrated that glutamatergic antagonists attenuate the ability of dopamine uptake blockers to increase extracellular levels of dopamine in the striatum (27) and nucleus accumbens (28). As cocaine is believed to act in part by blocking the reuptake of dopamine, it is possible that an agent that is capable of blocking glutamate release could reduce the pleasurable and reinforcing effects of cocaine.
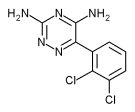
Lamotrigine is an anticonvulsant that is indicated as adjunctive therapy in the treatment of partial seizures in adults. It is a triazine derivative chemically unrelated to any other antiepileptic drug. The precise mechanism(s) of action of lamotrigine are unknown. Laboratory studies suggest that lamotrigine inhibits voltage-sensitive sodium channels., thereby stabilizing neuronal membranes and consequently decreasing presynaptic release of glutamate and aspartate (29, 30).
To our knowledge, only one study evaluating the use of lamotrigine for cocaine abuse has been conducted in human subjects. This study, conducted by Margolin and colleagues (31), showed that lamotrigine, given at a gradually increasing open-label dose over a period of 12 weeks to a sample of human immunodeficiency virus (HIV) seropositive patients, reduced cocaine use as determined by the decrease in percentage of cocaine-positive urine screens. The purpose of our study was to evaluate the effects of acute pretreatment with lamotrigine on the physiological and behavioral responses to intranasal cocaine in cocaine-dependent volunteers.
METHODS
Subjects
The study sample consisted of 12 non-treatment-seeking volunteers (8 male, 4 female), all of whom were hospitalized on a locked psychiatric unit at the Connecticut Mental Health Center. Each subject gave written informed consent prior to participation in any study procedure. The mean age of participants was 31.5 ([+ or -] 6.2) years. Eight subjects were African-American, two Hispanic, and two Caucasian. Diagnoses were established by semistructured clinical interview. All subjects met criteria for cocaine dependence (DSM-III-R), and minimum street cocaine use by history was at least 10 g during the preceding 3 months and laboratory confirmation of cocaine use by urine toxicology during the 3 weeks prior to entry. All subjects reported having experience with cocaine by the intranasal route; 83% had also used cocaine by smoking and 67% by the intravenous route. All subjects also met (DSM-III-R) criteria for ethanol abuse, and some subjects reported marijuana use, but the drug of choice for each subject was clearly cocaine. Subjects had a medical and psychiatric evaluation prior to enrollment in the study. There were no other comorbid medical or psychiatric diagnoses in the sample.
Treatments
Each subject had to complete the double-blind six experimental sessions over a 5-week inpatient stay. Study days occurred at least 4 days apart to allow elimination of residual lamotrigine. Drug identities for the 6 days, in random sequence, were as follows: placebo lamotrigine and placebo cocaine, lamotrigine 125 mg and placebo cocaine, lamotrigine 250 mg and placebo cocaine, placebo lamotrigine and cocaine 120mg/70 kg, lamotrigine 125 mg and cocaine 120 mg/70 kg, lamotrigine 250 mg and cocaine 120 mg/70 kg. The 125 and 250 mg doses of lamotrigine were selected to approximate the highest doses for which there were single-dose human subject safety data when the study was designed (120 and 240 mg) (32). Of the 12 subjects, 8 completed the six sessions. Four subjects completed one session and withdrew consent, citing a lack of willingness to complete the 5-week hospitalization.
Experimental Design
Subjects received oral lamotrigine and, 2 hours later, intranasal cocaine 120 mg/70 kg or placebo, consisting of lactose plus 4 mg cocaine. Cardiac monitoring was conducted for 4 hours following cocaine administration. An indwelling intravenous catheter was inserted for sampling of plasma cocaine levels at 15, 30, 60, and 120 minutes postadministration of cocaine. Samples taken at 60 minutes were also tested for lamotrigine concentration. However, for one subject, the 60-minute sample on the placebo cocaine/lamotrigine 250 mg per day was an sufficient quantity.
Behavioral Measures
During the study sessions, subjects responded to three mood state questionnaires: 100 mm Visual Analog Scales (VAS), the Profile of Mood States (POMS), and the Adjective Ratings Scale (ARS). The VAS (33) comprised 9 items (drug strength, good effects, bad effects, high, liking drug effect, rush, desire for cocaine, anxious/nervous, and relaxed/calm) anchored at 0 (not at all) and 100 (extremely or most ever). The ARS consists of 27 items (for example, active, alert, relaxed, etc.), with scores ranging from 0 (not at all) to 4 (extremely). The POMS is a 72-item scale with each item score ranging from 0 (not at all) to 4 (extremely). The 72 items generate 8 factors: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, confusion-bewilderment, friendliness, and elation. This version of POMS has been described by Fischman and Schuster (34) after the original 65 item test by McNair et al. (35). Ratings were completed before administration of lamotrigine (-130 minutes) and at 15, 45, 75, and 105 minutes after administration of cocaine in all subjects.
Data Analyses
Data were entered into a 2 x 3 x 5 repeated-measures analysis of variance (ANOVA) with cocaine versus placebo, lamotrigine dose (0, 125 mg, 250 mg), and time (-130, 15, 45, 75, and 105 minutes) as independent factors. For cardiovascular measures, data were analyzed at 15, 30, 45, 60, 75, and 120 minutes. Huynh-Feldt corrected values were reported when the Mauchly sphericity test was significant (36). Significant interactions and significant main effects were subjected to further post hoc analysis to determine the time points at which significant effects occurred. Student, single sample, or paired t tests, as appropriate, were performed on the summary variables "net cocaine response" and "net lamotrigine effect." The net cocaine response was defined as equal to (active cocaine time point - active cocaine baseline) -- (placebo time point -- placebo baseline). Similarly, a "net lamotrigine effect" was defined as equal to (active lamotrigine time point -- active lamotrigine baseline) -- (placebo time point -- placebo baseline). Significance was determined when p [is less than] .05 with a two-tailed test.
RESULTS
Plasma Levels
Plasma cocaine levels. The peak plasma cocaine levels were observed at 30 and 60 minutes after cocaine administration. At 30 minutes, mean + SD cocaine levels were 210 [+ or -] 107, 219 [+ or -] 103, and 235 [+ or -] 131 ng/ml for placebo, 125 mg lamotrigine, and 250 mg lamotrigine, respectively.
Plasma lamotrigine levels. As expected, plasma lamotrigine levels were significantly higher with the 250 mg close compared to the 125 mg dose on placebo cocaine days (2156 [+ or -] 1026 vs. 1054 [+ or -] 532 ng/ml, respectively, t = -5.27, df = 6, p = .002), as well as on active cocaine days (2726 [+ or -] 645 vs. 1328 [+ or -] 364 ng/ml, respectively, t = -11.78, df = 7, p = .001). Plasma lamotrigine levels were not significantly different on active cocaine days compared to placebo days.
Physiological Measures
Effects of cocaine alone. ANOVA revealed a significant cocaine x time interaction on heart rate [F(12,84) = 8.6, p = .001], systolic blood pressure [F(12,84) = 8.5, p = .001], and diastolic blood pressure [F(12,84) = 5.4, p = .001]. Post hoc t tests also revealed that cocaine, on placebo lamotrigine days, produced significant increases in heart rate at 15-, 30-, 45-, 60-, 75-, and 120-minute time points (p = .002, .001, .001, .002, .004, and .001, respectively) (see Table 1). Similarly, there was also a significant increase in systolic blood pressure at 15-, 60-, and 75-minute time points (p = .014, .034, .048, respectively). Cocaine alone, however, did not produce any significant change in diastolic blood pressure.
Table 1. Effects of Lamotrigine on the Net Cocaine Response(a) at Peak(b) (Mean [+ or -] SD) for Selected Measures
All comparisons across lamotrigine/placebo dose were n.s.
(a) Net cocaine response = (active cocaine time point -- active cocaine baseline) -- (placebo time point -- placebo baseline).
(b) "At peak" indicates the time point at which net cocaine response was maximal with placebo lamotrigine.
Effects of lamotrigine alone. ANOVA revealed a significant main effect of lamotrigine only on systolic blood pressure [F(2,14) = 7.3, p = .007]. However, post hoc t tests revealed no significant lamotrigine effect on this measure at any individual time point.
Effects of lamotrigine on responses to cocaine. ANOVA did not reveal a significant lamotrigine x cocaine x time interaction for any cardiovascular measure. Net cocaine responses at peak are shown in Table 1.
Behavioral Measures
Responses to cocaine.
Visual Analog Scale: ANOVA revealed a significant cocaine x time interaction for 8 of 9 VAS measures (all except relaxed/calm), as well as significant main effects of time and cocaine on 7 of 9 measures. For example, analysis of the measure high revealed a significant main effect of time [F(4,28) = 19.2, p = .001], a significant main effect of cocaine [F(1,7) = 22.3, p = .002], and a significant cocaine x time interaction [F(4,28) = 19.6, p = .001]. Figure 1 displays data for high. Post hoc t tests on placebo lamotrigine days revealed that cocaine produced significant placebo-corrected increases at one or more time points for 5 VAS measures. Analysis revealed significant net cocaine responses at 15, 45, and 75 minutes on the measures high (p = .007, .007, and .013, respectively) and good effects (p = .007..017, and .035, respectively). Similarly, significant net cocaine responses were seen at 15 and 45 minutes on the measures liking drug effect (p = .019 and .009) and rush (p = .001 and .020). Responses on the measure of drug strength were significantly higher on all four time points (p = .004, .007, .010, and .015, respectively).
[Figure 1 ILLUSTRATION OMITTED]
Adjective Ratings Scale: ANOVA revealed a significant cocaine x time interaction for 7 of 27 ARS measures, including nervous/afraid, racing heart, relaxed, clumsy, dazed, shaky, and spaced out. Post hoc t tests on placebo lamotrigine days revealed significant net cocaine responses only on racing heart at 15 minutes (p = .026).
Profile of Mood States: ANOVA revealed a significant cocaine x time interaction for 2 of 8 POMS factors: tension-anxiety [F(4,28) = 9.3, p = .002], and confusion [F(4,28) = 5.9, p = .001]. However, post hoc t tests did not reveal any significant effect of cocaine on these factors at any individual time point.
Effects of lamotrigine.
Visual Analog Scale: The ANOVA main effects of lamotrigine were significant only for relaxed/calm [F(2,14) = 6.5, p = .01], and none of the interactions of lamotrigine x time were significant. However, post-hoc t tests revealed that neither dose of lamotrigine had any significant effect on the net cocaine response at any time point on this measure.
Adjective Ratings Scale: ANOVA revealed a significant main effect of lamotrigine only on relaxed [F(2,12) = 5.5, p = .02]. Post hoc t tests on placebo cocaine days also revealed a net lamotrigine effect on relaxed. Analysis showed that lamotrigine, at 125 mg, produced a significant placebo-corrected increase from baseline at 75 minutes on relaxed (p = .02). Similarly, at 250 mg, there was a significant placebo-corrected increase from baseline at 45 minutes on this measure (p = .03).
Profile of Mood States: ANOVA did not reveal a significant lamotrigine x time interaction for any POMS factor.
Effects of lamotrigine on responses to cocaine.
Visual Analog Scale: ANOVA did not reveal a significant lamotrigine x cocaine x time interaction for any VAS measure. Net cocaine responses, for selected measures, at the time point producing a peak mean response on placebo lamotrigine days are shown in Table 1.
Adjective Ratings Scale: ANOVA revealed a significant lamotrigine x cocaine x time interaction only for I of 27 ARS measures, lazy [F(8,48) = 2.7, p = .02]. At the dose of 125 mg, lamotrigine produced significant increases in the net cocaine response at 15 minutes on lazy (p = .017), but no significant effect at 250 mg.
Profile of Mood States: ANOVA did not reveal a significant lamotrigine x cocaine x time interaction for any POMS factor.
DISCUSSION
Cocaine produced the expected increases on subjective measures reflecting pleasurable mood effects and liking for the drug in this study. Significant increases were also observed on cardiovascular variables. Lamotrigine alone produced a mild relaxing effect, but no behavioral effect that would appear to substitute for those of cocaine. When given together with cocaine, lamotrigine did not alter the variables thought to relate to reducing the reinforcing properties of cocaine, reducing cocaine craving, or increasing the aversive properties of cocaine. Taken together, these findings are not supportive of an ability of lamotrigine to reduce cocaine use in clinical practice.
These results stand somewhat in contrast to those of our previous study, which found that the rate of cocaine-positive urines decreased by 50% in cocaine-abusing patients during chronic administration of open-label lamotrigine 300 mg/day, as compared to the predrug baseline, when the lamotrigine was initiated with a slow induction schedule (31). One possibility is that lamotrigine may have induced a state of relaxation in the patients in that study, similar to the effect seen here, and that patients may be less likely to use cocaine when in this relaxed state. A somewhat similar effect of lamotrigine has been seen in epileptic patients in two studies (37, 38), in which subjects reported being significantly happier and having a higher degree of mastery or perceived internal control while receiving lamotrigine. Another difference between the two studies that might explain the discrepant findings is that our previous study administered lamotrigine over 12 weeks as opposed to the single doses employed here. Because of the chronic dosing, it is likely that the levels attained in the previous study were somewhat higher than in the current one, although with chronic dosing lamotrigine has been shown to induce its own metabolism, which would reduce the effect of chronic dosing on increasing the plasma level. Plasma levels of lamotrigine were not done in our previous study. We are unaware of studies investigating differential neurobiological effects with chronic versus acute administration of drugs that reduce glutamate function. In addition, the previous study had no placebo control.
Another aspect of lamotrigine is worth mentioning here. Since our study was designed, some authors have disputed whether lamotrigine suppresses EAAs more than other neurotransmitters (39). Lamotrigine did not attenuate another phenomenon thought to be EAA mediated in humans, opiate withdrawal (40).
The data do not indicate any specific safety concerns in coadministering lamotrigine and cocaine. No significant drug interactions occurred between the two drugs. Also, no serious adverse effects occurred in response to lamotrigine alone.
This was a preliminary study with several limitations, including the small sample size. We also administered cocaine via the intranasal route, which may be less optimal in such experimental situations because lower plasma cocaine levels are achieved and pharmacological effects are slow to appear and have lesser magnitude than observed in connection with the intravenous or smoking route (41). Last, we suggest that a chronic dosing schedule of lamotrigine be employed, perhaps using larger doses. Studies employing chronic dosing may also evaluate the potential neuroprotective effects of lamotrigine (42, 43) in cocaine addicts. Future research should also investigate medications other than lamotrigine to determine whether other drugs that reduce EAA function may produce beneficial effects on cocaine use in humans.
ACKNOWLEDGMENT
This research was supported by NIDA grants P50-DA04060, P50-DA09250, and K02-DA00112.
REFERENCES
(1.) National Institute on Drug Abuse, Facts About Cocaine Abuse and Treatment, NIDA Notes 10(5), Author, Rockville, Maryland, 1995.
(2.) Covi, L., Montoya, I. D., Hess, J. M., et al., Desipramine for treatment of cocaine dependence: randomized, double-blind, placebo-controlled study, ACNP Ann. Meeting Abs. 173 (1993).
(3.) Covi, L., Hess, J. M., Kreiter, N. A., et al., Desipramine and counselling for treatment of cocaine dependence: a controlled study, NIDA Res. Monogr. 153:309 (1994).
(4.) Walsh, S. L., Preston, K. L., Sullivan, J. T., et al., Fluoxetine alters the effects of intravenous cocaine in humans, J. Clin. Psychopharmacol. 14:396-407 (1994).
(5.) Walsh, S. L., Sullivan, J. T., and Bigelow, G. E., Fluoxetine effects on cocaine responses: a double-blind assessment, NIDA Res. Monogr. 153:310 (1994).
(6.) Batki, S. L., Washburn, A. M., Delucchi, K., et al., A controlled trial of fluoxetine in crack cocaine dependence, Drug Alcohol Depend. 41:137-142 (1996).
(7.) Preston, K. L., Sullivan, J. T., Strain, F. C., et al., Effects of cocaine alone and in combination with bromocriptine in human cocaine abusers, J. Pharmacol. Exp. Ther. 262:279-291 (1992).
(8.) Weddington, W. W., Brown, B. S., Haertzen, C. A., et al., Comparison of amantadine and desipramine combined with psychotherapy for treatment of cocaine dependence, NIDA Res. Monogr. 95:483-484 (1989).
(9.) Alterman, A. I., Droba, M., Antelo, R. E., et al., Amantadine may facilitate detoxification of cocaine addicts, Drug Alcohol Depend. 31:19-29 (1992).
(10.) Margolin, A., Kosten, T. R., Avants, S K., et al., A multicenter trial of bupropion for cocaine dependence in methadone-maintained patients, Drug Alcohol Depend. 40:125-131 (1995).
(11.) Sherer, M. A., Kumor, K. M., and Jaffe, J. H., Effects of intravenous cocaine are partially attenuated by haloperidol, Psychiatry Res. 27:117-125 (1989).
(12.) Stine, S., Krystal, J., Kosten, T., et al, Mazindol treatment of cocaine abusers, ACNP Ann. Meeting Abs. 228 (1992).
(13.) Preston, K. L., Sullivan, J. T., Berger, P., et al., Effects of cocaine alone and in combination with mazindol in human cocaine abusers, J. Pharmacol. Exp. Ther. 267:296-307 (1993).
(14.) Hatsukami, D., Keenan, R., Halikas, J., et al., Effects of carbamazepine on acute responses to smoked cocaine-base in human cocaine users, Psychopharmacology 104:120-124 (1991).
(15.) Halikas, J. A., Crosby, R. D., Pearson, V. L., et al., A randomized double- blind study of carbamazepine in the treatment of cocaine abuse, Clin. Pharmacol. Ther. 62:89-105 (1997).
(16.) Montoya, I. D., Levin, F. R., Fudala, F. J., et al., Double-blind study with carbamazepine for treatment of cocaine dependence, ACNP Ann. Meeting Abs. 173 (1993).
(17.) Kranzler, H. R., Bauer, L. O., Hersh, D., et al., Carbamazepine treatment of cocaine dependence: a placebo-controlled trial [see comments], Drug Alcohol Depend. 38:203-311 (1995).
(18.) Kosten, T., Silverman, D. G., Fleming, J., et al., Intravenous cocaine challenges during naltrexone maintenance: a preliminary study, Biol. Psychiatry 32:543-548 (1992).
(19.) Carroll, K., Ziedonis, D., O'Malley, S., et al., Pharmacologic interventions for abusers of alcohol and cocaine: disulfiram versus naltrexone, Am. J. Addict. 2:77-79 (1993).
(20.) Hameedi, F. A., Rosen, M. I., McCance-Katz, E. F., et al., Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans, Biol. Psychiatry 37:560-563 (1995).
(21.) Pulvirenti, L., Sung, R., and Koob, G. F., Microinjection of NMDA but not quisqualate receptor antagonists into the nucleus accumbens modulates intravenous cocaine self-administration in rats, Soc. Neurosci. Abs. 15:1098 (1989).
(22.) Pulvirenti, L., Swerdlow, N. R., and Koob, G. F., Nucleus accumbens NMDA antagonist decreases locomotor activity produced by cocaine, heroin or accumbens dopamine, but not caffeine, Pharmacol. Biochem. Behav. 40:841-845 (1991).
(23.) Johnson, M., Bush, L. G., Gibb, J. W., et al., Role of N-methyl-D-aspartate (NMDA) receptors in the response of extrapyramidal neurotensin and dynorphin A systems to cocaine and GBR 12909, Biochem. Pharmacol. 41:649-652 (1991).
(24.) Torres, G., and Rivier, C., Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists, Brain Res. Bull. 30:173-176 (1993).
(25.) Rockhold, R. W., Oden, G., Ho, I. K., et al., Glutamate receptor antagonists block cocaine-induced convulsions and death, Brain Res. Bull. 27:721-723 (1991).
(26.) Witkin, J. M., and Tortella, F. C., Modulators of N-methyl-D-aspartate protect against diazepam-or phenobarbital-resistant cocaine convulsions, Life Sci. 48:L51-L56 (1991).
(27.) Moghaddam, B., and Bolinao, M. L., Glutamatergic antagonists attenuate ability of dopamine uptake blockers to increase extracellular levels of dopamine: implications for tonic influence of glutamate on dopamine release, Synapse 18:337-342 (1994).
(28.) Pap, A., and Bradberry, C. W., Excitatory amino acid antagonists attenuate the effects of cocaine on extracellular dopamine in the nucleus accumbens, J. Pharmacol. Exp. Ther. 274:127-133 (1995).
(29.) Leach, M. J., Marden, C. M., and Miller, A. A., Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action, Epilepsia 27: 490-497 (1986).
(30.) McGeer, E. G., and Zhu, S. G., Lamotrigine protects against kainate but not ibotenate lesions in rat striatum, Neurosci. Lett. 112:348-351 (1990).
(31.) Margolin, A., Avants, S. K., DePhillips, D., et al., A preliminary investigation of lamotrigine for cocaine abuse in HIV-seropositive patients, Am. J. Drug Alcohol Abuse 24:85-101 (1998).
(32.) Cohen, A. F., Ashby, L., Crowley, D., et al., Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam, Br. J. Clin. Pharmacol. 20:619-629 (1985).
(33.) Van Dyke, C., Ungerer, J., Jatlow, P., et al., Intranasal cocaine: dose relationships of psychological effects and plasma levels, Int. J. Psychiatry Med. 12:1-13 (1982).
(34.) Fischman, M. W., and Schuster, C. R., Cocaine effects in sleep-deprived humans, Psychopharmacology 72:1-8 (1980).
(35.) McNair, D. M., Lorr, M., and Droppleman, L. F., Profile of Mood States (Manual), Educational and Industrial Testing Service, San Diego, California, 1971.
(36.) Stevens, J., Applied Multivariate Statistics for the Social Sciences, 2nd ed., Lawrence Erlbaum Associates, Hillsdale, New Jersey, 1992, p. 448.
(37.) Smith, D., Baker, G., Davies, G., et al., Outcomes of add-on treatment with lamotrigine in partial epilepsy, Epilepsia 34:312-322 (1993).
(38.) Smith, D., Chadwick, D., Baker, G., et al., Seizure severity and the quality of life, Epilepsia 34:S31-S35 (1993).
(39.) Waldmeier, P. C., Baumann, P. A., Wicki, P., et al., Similar potency of carbamazepine, oxcarbazepine, and lamotrigine in inhibiting the release of glutamate and other neurotransmitters, Neurology 45:1907-1913 (1995).
(40.) Rosen, M. I., Pearsall, H. R., and Kosten, T. R., The effect of lamotrigine on naloxone-precipitated opiate withdrawal, Drug Alcohol Depend. 52:173-176 (1998).
(41.) Cone, E. J., Pharmacokinetics and pharmacodynamics of cocaine, J. Anal. Toxicol. 19:459-478 (1995).
(42.) Shuaib, A., Mahmood, R. H., Wishart, T., et al., Neuroprotective effects of lamotrigine in global ischemia in gerbils. A histological, in vivo microdialysis and behavioral study, Brain Res. 702: 199-206 (1995).
(43.) Crumrine, C., Bergstrand, K., Cooper, A. T., et al., Lamotrigine prevents brain damage resulting from global ischemia caused by cardiac arrest with resuscitation, Soc. Neurosci. Abs. 23:2433 (1997).
Lynn C. Winther, M.D.(1,2,3),(*) Rehan Saleem, M.D.(2,3) Elinore F. McCance-Katz, M.D., Ph.D.(1,3,4) Marc I. Rosen, M.D.(1,3,4) Faiq A. Hameedi, M.D., M.P.H.(1,2,3) H. Rowland Pearsall, M.D.(2,3) Peter I. Jatlow, M.D.(1) Thomas R. Kosten, M.D.(1,3,4) Scott W. Woods, M.D.(2,3),([dagger])
(*) Dr. Winther is currently in the Department of Psychiatry, Albert Einstein School of Medicine, New York, New York.
([dagger]) To whom reprint requests should be addressed at Yale University School of Medicine, 34 Park Street, New Haven, CT 06519. Telephone: (203) 789-6871.
(1) Substance Abuse Division
(2) Connecticut Mental Health Center
(3) Department of Psychiatry Yale University School of Medicine
(4) Connecticut VA Healthcare System New Haven, Connecticut
COPYRIGHT 2000 Marcel Dekker, Inc.
COPYRIGHT 2001 Gale Group