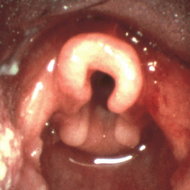
We describe a patient who, 4 years after a radical neck dissection and radiotherapy, presented with obstructive sleep apnea; upon bronchoscopy, he was found to have acquired laryngomalacia. Inspiration induced upper airway obstruction due to a large flaccid epiglottis, large aryepiglottic folds, and edema of the supraglottic area. We suggest that acquired laryngomalacia can lead to obstructive sleep apnea. Patients with obstructive sleep apnea after radical neck dissection need to be evaluated for laryngomalacia with fiberoptic laryngobronchoscopy. Examination of the upper airway is useful to determine the nature and extent of any upper airway collapse.
(Chest 1994; 106:1898-1899)
OSA=obstructive sleep apnea
Key words: laryngomalacia; obstructive sleep apnea; upper airway
The mechanism of obstructive sleep apnea (OSA) appears to be multifactorial. Anatomic narrowing, increased compliance, altered reflexes, and inspiratory pharyngeal muscle dysfunction may all play a role in the pathophysiology of OSA.(1) Thus, structural alterations as well as the balance between pharyngeal dilating and collapsing forces are important in maintaining upper airway patency. The collapsing segment itself may vary in location from the oropharynx to the hypopharynx.(1) Although the incidence is low, several upper airway abnormalities can produce the OSA syndrome. Examination of the nasal passages and oropharynx is performed routinely to detect nasal obstruction, enlarged tonsils or adenoids, macroglossia, and hypopharyngeal masses.
In infants and children, OSA symptoms have been described with congenital laryngomalacia, an entity characterized by inspiratory stridor due to a flaccid epiglottis, redundant aryepiglottic folds, or hypotonia of the larynx. Belmont and Grundfast(2) evaluated 30 such infants with congenital laryngeal stridor. Endoscopy characteristically showed flaccid supraglottic structures and medial prolapse of the arytenoid folds or of the epiglottis on inspiration. Seven of these infants had OSA.
We describe an adult patient who had had a left radical neck dissection followed by radiotherapy and who years later presented with OSA. Endoscopy revealed flaccid supraglottic tissues. Airflow-induced collapse of the epiglottis into the laryngeal inlet causing intermittent obstruction was also noted. The term acquired laryngomalacia has been used to describe the preceding findings.(3) Our patient suggests that acquired laryngomalacia due to neck surgery with radiotherapy may lead to OSA.
CASE REPORT
A 42-year-old man was admitted to the hospital with complaints of mild swelling of the lips, face, and tongue over 3 days. He had been diagnosed as having carcinoma of the anterior floor of the mouth 4 years before hospital admission. At that time, he underwent a left modified radical neck dissection with removal of the tumor mass and the inner table of the mandible. During the neck dissection, the omohyoid, geniohyoid, myelohyoid, and genioglossus muscles were sacrificed. A temporary tracheostomy was done and this closed with time. The patient also received radiation therapy using a 6-MeV linear accelerator. A total dose of 60 Gy was delivered to the midline of the neck and a dose of 50 Gy was delivered to the lower neck. Several months after radiation therapy, he was started on a regimen of levothyroxine (120 [micro]g each day). He quit smoking. The patient consumed a moderate amount of alcohol.
Examination at the time of hospital admission showed an oriented afebrile patient with a weight of 105 kg and height of 185 cm. The left side of the neck was deformed and there was mild edema of the lower lip and left side of the face. No lymph nodes were palpable. A mild inspiratory stridor was noted. The chest, cardiovascular, and results of the rest of the examination were normal. Results of routine laboratory tests as well as thyroid function tests were normal. Spirometry showed a FE[V.sub.1] of 3.82 L (83 percent of predicted) and a FVC of 4.29 L (79 percent of predicted). The flow volume curve, including the inspiratory limb, was normal. Chest radiograph was normal. A computed tomographic scan of the neck and chest showed no masses. Angioedema was suspected clinically and the patient was treated with antihistamines and steroids without any benefit.
On the ward, the patient was noted to snore and have apneic events. On further questioning, he admitted to mild daytime sleepiness. Consequently, a nocturnal polysomnography study was performed. This study showed a an apnea + hypopnea index of 67 events per hour of sleep. The apneas were associated with oxygen desaturations from a baseline of 95 percent to a minimum level of 60 percent. One hundred fifteen episodes of oxygen desaturation below 85 percent were noted. Sleep desaturation and apneic episodes were reduced when the patient was on his left side. A trial of nasal continuous positive airway pressure of 7.5 cm reduced the apnea + hypopnea index to 7.1 events per hour, but the patient complained of nasal congestion and refused to wear the mask. Because the patient also complained of choking and coughing while eating, a video fluoroscopic modified barium swallow study was performed. This showed considerable residue in the valleculae. The epiglottis appeared to be enlarged and to descend on inspiration causing partial airway obstruction.
A flexible fiberoptic bronchoscopy was performed nasally after local anesthesia, first without sedation and after the first examination mild sedation was induced with intravenous midazolam. The oropharynx and nasopharynx appeared normal with no undue collapse. The epiglottis was large, edematous, and flaccid. It obscured the glottis and vibrated vigorously with respirations. Visualization of laryngeal structures showed large redundant aryepiglottic folds and edematous mobile mucosa. The enlarged aryepiglottic folds prolapsed with inspiration into the glottic space. The remaining tracheobroncheal examination showed normal findings except for mild narrowing of the proximal trachea.
DISCUSSION
The most striking findings in our patient consisted of flaccid supraglottic structures associated with obstruction of the glottis during inspiration. The flaccidity of the supraglottic area may have resulted from the radical neck dissection and radiation therapy. The lymphatic destruction and sacrifice of the supraglottic muscles might have compromised the size and stability of the upper airway. Chronic aspiration might also have contributed to the laryngeal damage but this was not documented well. Our findings are similar to those observed by Woo(3) and Kletzker and Bastian.(4) Their patients had upper airway obstruction either due to epiglottic prolapse or abnormally mobile supraglotic mucosa. Three of their patients had had neck surgery as well as complaints and upper airway obstructive symptoms consistent with sleep apnea. Staged radical neck dissection after radiotherapy to the neck may also lead to acute airways obstruction.(5) Sleep apnea has been reported in two other patients as a result of radical neck surgery and radiotherapy.(6)(7)
In conclusion, it appears that supraglottic airway obstruction may occur in some patients after radical neck dissection and radiation therapy. These procedures may derange supraglottic anatomy as well as neuromuscular control and lead to upper airways obstruction and OSA. With laryngomalacia, management is surgical(3)(8) and consists of laser epiglottectomy or supraglottoplasty together with resection of arytenoid mucosa or alternatively a tracheostomy. Our patient refused these procedures. He continued to report only mild daytime sleepiness 1 year after the diagnosis was made, suggesting that in his case, the process was not progressive.
REFERENCES
(1)Hudgel DW. Mechanisms of obstructive sleep apnea. Chest 1992; 101:541-49
(2)Belmont JR, Grundfast K. Congenital laryngeal stridor (laryngomalacia): etiologic factors and associated disorders. Ann Otol Rhinol Laryngol 1984; 93:430-37
(3)Woo P. Acquired laryngomalacia: epiglottis prolapse as a cause of airway obstruction. Ann Otol Rhinol Laryngol 1992; 101:314-20
(4)Kletzker GR, Bastian RW. Acquired airway obstruction from histologically normal, abnormally mobile supraglottic soft tissues. Laryngoscope 1990; 100:375-79
(5)Brown AMS, Millar BG. Acute upper airway obstruction following staged bilateral radical neck dissections in previously irradiated patients. Br J Oral Maxillofac Surg 1990; 28:272-74
(6)Baker SR, Ross J. Sleep apnea syndrome and supraglottic edema. Arch Otolaryngol 1980; 106:486-91
(7)Polnitsky CA, Sherter CB, Sugar JO. Irradiation-induced fibrosis of the neck and sleep apnea. Arch Otolaryngol 1981; 107:629-30
(8)Holinger LD, Konior RJ. Surgical management of severe laryngomalacia. Laryngoscope 1989; 99:136-42
COPYRIGHT 1994 American College of Chest Physicians
COPYRIGHT 2004 Gale Group