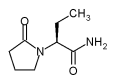
Use of antiepileptic drugs (AEDs) is common in older patients, especially those in nursing homes (NH). Recent studies have reported that approximately 10% of NH residents in the United States are receiving AEDs. (1,2) Approximately 7% are being treated with AEDs on admission, and nearly 3% have an AED added after admission. (3) Many older patients and nearly all NH residents on AED therapy are also taking other drugs for comorbid conditions. A large population study reported that the average older NH patient takes six medications concomitantly in addition to AEDs, greatly increasing the risk for side effects and drug interactions. (2)
AEDs can be classified as those available before 1980 (carbamazepine, phenytoin, phenobarbital, and valproate) or "first generation," and those available after 1993 (felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, tiagabine, topiramate, and zonisamide) or "second generation" AEDs. A recently published survey using 1999 data indicates that the most commonly used AEDs are the first generation AEDs, initiated prior to and after NH admission. (3) However, first generation AEDs have properties that may contribute to their propensity to cause side effects in older adults.
Complications from first generation drugs also may add substantially to the cost of care for older patients. (4) Improved quality of life and reduced overall care costs may be possible with careful selection of AEDs. This paper will review the profiles of first and second generation AEDs and suggest means to improve care in older adults with epilepsy.
Methods
This paper reflects the presentations and deliberations of a panel of neurologists with expertise in the treatment of older epilepsy patients, long-term care (LTC) medical directors, and consultant pharmacists to the LTC facilities. Until recently there has been little interest or research in this area, but data are emerging from the Veterans Affairs (VA) Cooperative Study, (5) a National Institutes of Health (NIH) supported project, (4) and other studies such as the KEEPERTM (6) and the STEPS (7) trials. Results from these trials are leading to reconsideration of the use of AEDs. This panel was stimulated by the need to incorporate the new information into a rational framework to select the best AED for the older population.
Seizures in older adults
The older population with epilepsy comprises those with long-standing epilepsy and those with relatively new-onset seizures. Annual incidence of seizures in the geriatric population is greater than in any other age group, greatly surpassing that in children and younger adults. (8,9) Moreover, seizures in older adults have unique characteristics, and older people differ from their younger counterparts with respect to physiology, associated diseases, medication use, and other features such as metabolism, drug absorption and elimination, low- or below-normal albumin levels, and changes in drug tolerance.
Most seizures in older patients are caused by a focal area of damage to the brain, such as stroke, tumor, injury, or degenerative disorder. The most common seizure types are partial seizures. These can be simple partial (no loss of awareness) or complex partial (loss of awareness). Complex partial seizures are the most common seizure type, accounting for nearly 40% of seizures in the older population. (9) Simple and complex may spread and develop into generalized tonic-clonic seizures. All major AEDs, both the first and second generation AEDs (with the exception of ethosuximide), have an FDA indication for use in complex partial seizures.
Choosing an ideal agent
Studies indicate that all FDA-approved AEDs have similar efficacy, therefore, selection of AED therapy for older patients should consider tolerability. When evaluating AEDs for this population, an agent's potential for drug interactions should play a major role in treatment selection. The prime consideration when evaluating the appropriate agent is the patient's functional status.
Pharmacokinetic and pharmacodynamic considerations that help define the characteristics of the ideal AED for the older patient include:
* lack of side effects or toxicity
* absorption (complete oral absorption, water soluble)
* no drug-drug interactions
* long half-life
* no hepatic metabolism or enzyme induction/inhibition
* no active metabolites
* readily excreted
* minimal protein binding
* no effects on bone loss
* reasonably priced. (10)
Older persons experience alterations in gastrointestinal drug absorption due to changes in gastric pH, gastric emptying, or intestinal motility. (11) Absorption of the AEDs that are highly insoluble (eg, phenytoin) complicates dosing due to increased variability in absorption. (4) Lamotrigine is more soluble than first generation AEDs. (12) Levetiracetam is highly soluble and readily absorbed. (13) Gabapentin is very water soluble, but relies on a rate-limiting transport system for absorption, and, as a result, displays dose-dependent oral absorption. (14)
Older persons experience a decline in liver metabolic capacity. An AED not substantially metabolized by the liver would have obvious benefits in this population. Renal function also will decline during the natural course of aging, so changes in the clearance of these medications would also be expected. As shown in table 1, many of the first generation AEDs undergo hepatic metabolism. Second generation agents are primarily eliminated by renal clearance with no or minimal hepatic metabolism.
Evidence exists that, in general, second generation AEDs are as efficacious as first generation AEDs. A subgroup analysis of the KEEPER study, which assessed the use of levetiracetam as adjunctive therapy for partial-onset seizures in the age 65 population, showed that 76.9% of the patients overall had a >50% reduction in seizure frequency, and 40% had a 100% response, or total control of seizures for the duration of the study. (6) A similar earlier study, the Study of Titration to Effect Profile of Safety (STEPS) trial with gabapentin, also demonstrated similarly good efficacy in the older cohort of the study. (7) Equally important, both gabapentin and levetiracetam were well tolerated in the older population even as additional adjunctive therapy. In both studies, the older patients had long-standing refractory epilepsy requiring treatment with multiple AEDs. According to results from some small studies, levetiracetam also as monotherapy appears to provide good seizure control in older patients with epilepsy. (15)
The main differences between the first and second generation agents are the side-effect profiles, protein binding, and drug interactions. (16) Table 1 shows the comparative pharmacokinetics of the most commonly used first generation and second generation agents. Table 2 lists the drug interaction profiles of these agents. (16)
A multicenter, double-blind, randomized comparison study between lamotrigine and carbamazepine in older patients (mean age 77) with newly diagnosed epilepsy living in the United Kingdom, showed that the main difference between the groups was the rate of dropout due to adverse events (lamotrigine 18% versus carbamazepine 42%). (12)
The VA Cooperative Study #428, an 18-center, parallel, double-blind trial on the use of gabapentin, lamotrigine, and carbamazepine in patients age 60 and older, presented at the American Epilepsy Society meeting last December also illustrated that tolerability was a key factor in appropriate AED selection for this population. (5)
Therapeutic range
Therapeutic range, when applied to first generation AEDs, is relied upon to provide a range where efficacy can be expected and side effects tolerated; with the second generation agents, there is less experience with levels. In older patients, however, reliance on the established therapeutic range with first generation AEDs may be problematic. Increasing evidence indicates that older adults do not tolerate total serum levels of phenytoin in the mid- to high-therapeutic range and may develop typical side effects, such as ataxia and dizziness. Side effects, which may be promptly noted and reported in younger patients, may go unnoticed or underreported in older patients, specifically in NH populations. Similarly, the tremor produced by high levels of valproate or the cognitive or sedative effects of AEDs may be less readily recognized in the institutionalized population.
Variability of levels is another concern with the first generation AEDs. A recent study found variability across extended periods of time in total phenytoin concentrations in patients who had no change in dose or other factors. (4) This may also be the case for other water-insoluble AEDs such as carbamazepine; studies are in progress.
Chronic side effects
Osteoporosis, weight gain, gingival hyperplasia, and peripheral neuropathies can be caused by some of the first generation agents, but current data do not show this to be true for the second generation AEDs (table 3). These chronic side effects should be considered when selecting an agent because treatment is often lifelong, particularly in the patient with late-onset symptomatic epilepsy. Osteoporosis is a concern in older patients; hip fractures are a major cause of morbidity in this population. Older patients on AED therapy may be at especially high risk for osteoporosis and, most importantly, fractures. A study of 345 patients on AED therapy found that they had twice the risk for fracture as did controls. (17) AEDs cause bone loss by several different mechanisms, including increased metabolism of 25-OH vitamin D, hypocalcemia leading to secondary hyperparathyroidism without decreased vitamin D status, and increased bone turnover without a decrease in vitamin D status. Also, side effects of AED therapy such as somnolence, dizziness, and gait disturbances increase the propensity to fall and sustain a fracture. Recent evidence suggests phenytoin, carbamazepine, and valproate may be associated with bone loss in both older and younger patients with epilepsy. Emerging data from recent studies indicate that the second generation AEDs may have little contribution to osteoporosis, which may be related to their lack of enzyme-inducing activity.
Because the clinical and economic impact of fractures is substantial, fracture prevention protocols should be in place, and alternatives to enzyme-inducing AEDs known to have deleterious effects on bone should be considered, especially in patients with osteoporosis, osteomalacia, and secondary hyperparathyroidism.
Weight gain is another area of concern because of its association with the risk of diabetes and cardiovascular disease, which is significant in older adults who are at greater risk for these diseases. In a VA Cooperative study, (18) weight gain was the most common side effect reported by patients on treatment regimens of carbamazepine or valproate. Severe weight loss caused by AEDs in the older patient can also be a concern. Of the newer agents shown in table 3, a few promote some weight loss or are weight neutral.
Limitations of available AEDs
Phenytoin has been available for more than 65 years but is difficult to use because of its non-linear pharmacokinetics (ie, a small increase in dose can lead to a large increase in serum level), narrow therapeutic range, and its drug-drug interactions. (19) Phenytoin may not be the best choice when certain comedications or comorbidites are present. Drug interactions and side effects have been described when phenytoin is prescribed with drugs for hypertension, diabetes, arthritis, and oral coagulation, and in persons with a history of alcoholism, liver disease, or cerebral and cerebellar atrophy. Ataxia can be seen at serum concentrations considered therapeutic in younger patients; this side effect may signify that (a) the older patient is not tolerating the drug well at that level, and (b) the addition of another highly protein bound drug (eg, valproate) may worsen the patient's symptoms. Toxicity may not be apparent from serum monitoring of total phenytoin concentrations because only the free fraction of phenytoin, not the total serum level, is increased. The patient may appear to have reached a therapeutic level, when, in fact, the free fraction is significantly elevated. Because older patients often have a higher baseline free fraction, accurately measuring total concentrations may be misleading. In addition, the nonlinear transition to toxic levels is steeper in the older patient compared with younger individuals.
Carbamazepine is commonly used in the treatment of partial onset seizures. (18) However, carbamazepine has many drug-drug interactions (19) and a side-effect profile that can include blurred or double vision, ataxia, tremor, and gastrointestinal symptoms. It is also associated with hyponatremia, especially when used with sodium-depleting antihypertensive agents.
Generally, avoid phenobarbital use in older patients because it causes sedation, depression, cognitive decline, and has many drug interactions.
Of the older AEDs, valproate is the only broad spectrum agent, whereas several of the newer agents (lamotrigine, levetiracetam, topiramate, and zonisamide) appear to have broad spectrum efficacy.
AED opportunities
When the clinician recognizes that an older patient is intolerant of first generation agents, switching to the second generation AEDs should be considered. Although the initial change may involve the addition of the new agent to the first generation drug, monotherapy should always be a goal in older patients.
Many second generation AEDs have simplified pharmacokinetics. With the exception of felbamate, which is no longer commonly used due to safety concerns, all newer AEDs have fewer effects on other medications than do phenytoin, carbamazepine, and phenobarbital. Gabapentin and levetiracetam have the fewest drug interactions of the newer AEDs, with no significant effects on other medications (including other AEDs) and no effects of other medications on their levels or metabolism. Second generation AEDs metabolized by the liver (eg, lamotrigine, tiagabine, and zonisamide) may have reduced levels when given with enzyme inducers. Also, oxcarbazepine can inhibit the metabolism of phenytoin.
Many second generation AEDs have safety profiles better than those of the first generation. None of these AEDs (with the exception of felbamate) have had associated liver or bone marrow toxicity, and the numbers of exposed patients is now sufficient to establish this comfort level. In the institutionalized population, allergic reactions should be readily noted and medications adjusted. Of the newer AEDs, gabapentin and levetiracetam have a risk for all rashes of only about 1% and have not been associated with serious allergic reactions.
Lamotrigine can cause serious allergic reactions (eg, Stevens-Johnson syndrome, toxic epidermolysis), but with slow titration at introduction, incidence of these serious side effects is greatly reduced.
Oxcarbazepine is a congener of carbamazepine, having no epoxide metabolite, and less hepatic induction. Aplastic anemia and agranulocytosis seen with carbamazepine have not been seen with oxcarbazepine, which does not depress white cell counts. However, incidence of hyponatremia with oxcarbazepine is greater than with carbamazepine, and older patients are typically more predisposed to hyponatremia. (20)
Many older patients may be sensitive to the cognitive side effects of AEDs. This may be due to baseline cognitive dysfunction, multiple medications, other concomitant medical problems, or the inherent sensitivity of older patients to such effects. Many of the newer AEDs, specifically gabapentin, lamotrigine, and levetiracetam, appear to have particularly favorable cognitive profiles compared with the first generation AEDs. Recent studies have compared gabapentin with carbamazepine in older patients and demonstrated significantly fewer cognitive side effects with gabapentin than with carbamazepine. (21) In a review of 3,252 persons involved in studies of levetiracetam for epilepsy and other conditions, levetiracetam was well tolerated by older adults. (22)
Dosing
Compliance is a potential challenge in older adults due to multiple medications, memory problems, and visual problems. In general, twice daily dosing is preferable. In LTC facilities, compliance may be less of an issue than with the community-dwelling residents, but reducing staff (eg, nurses) time spent administering multiple daily doses of medication to many patients may reduce errors and cost.
Only fosphenytoin converted to phenytoin, is available for intramuscular or intravenous (IV) use. Valproate is available as IV, and there is no marketed IV formulation for carbamazepine. Due to their solubility, some second generation agents can be crushed (topiramate, lamotrigine, levetiracetam) and put into a suspension, given via nasogastric tube (levetiracetam), or given rectally (zonisamide, lamotrigine, topiramate). An IV preparation of levetiracetam is presently undergoing equivalency testing and future availability is anticipated.
Of the newer AEDs, gabapentin and levetiracetam can be initiated at therapeutic doses. The other new AEDs typically need to be initiated at low doses and slowly titrated to minimize the risk of introduced side effects or allergic reactions. In addition, as noted earlier, older adults experience a reduction in renal function with age, but the broad therapeutic ratios of some of the newer AEDs make adjustments easy in these patients, even when significant renal insufficiency is present.
Implementation challenges
Advantages of using newer AEDs for treatment of seizures in older adults are many. Established algorithms, guidelines, and caregiver education are important to help overcome barriers to prescribing second generation AEDs to an AED-naive patient and to calm patient and caregiver reservations about switching from older to newer AEDs.
Which second generation agents are most appropriate? In the fall of 2000, a panel of expert epileptologists was asked which agent they would select as first-, second-, and third-line therapy in the symptomatic, localization-related, medically stable older man or woman. The experts selected gabapentin, lamotrigine, levetiracetam, and oxcarbazepine as the second generation agents they would use as first-line therapy.
What are the recommendations for changing the AED? Efficacy of the second generation agents has been found comparable to the first generation. The issue is how to best change the regimen to avoid any side effects, such as drug interactions, or breakthrough or worsening seizures. Crossover to a new agent takes time. The new agent should produce few, if any, drug interactions and be added to the ongoing AED program with the intent of reaching a dose that will be relatively protective before beginning dose reduction of the baseline drugs. No standard guidelines exist concerning how rapidly to withdraw the baseline AEDs. The authors consider a dose reduction of 25% per each step for each baseline drug to be reasonable and safe. The interval between steps should be at least five half-lives or a minimum of 1 week to allow steady state to occur. In addition, the inducing and inhibiting capabilities of the first generation AEDs may necessitate changes in co-medications during withdrawal of these AEDs from the regimen.
What about the cost of the newer agents? Cost must be considered but within the context of patients' quality of life and use of total healthcare resources. New AEDs are expensive--the first generation drugs are considerably less so. Most companies have devised programs that provide drugs at much reduced cost to those in need. Nonetheless, the current climate is one of severe difficulty for older patients. Our view, at this time, is that the higher cost of the second generation drugs may well be compensated for by fewer patient visits to the emergency department or the physician's office, and fewer hospitalizations, which are often associated with the managing of side effects, ordering additional lab tests to monitor drug levels and electroencephalograms to monitor seizure activity/control, and titrating drugs to effective dose levels common with the first generation AEDs.
Summary
Epileptic seizures in older patients are common neurologic problems, often requiring long-term treatment. Selection of the appropriate therapy should recognize that older adults in general have multiple comorbidities, are on multiple medications, have evidence of age-related changes in physiology, and may be more sensitive to toxic side effects of medications. First generation AEDs have a number of potential disadvantages in the treatment of older patients; many second generation AEDs avoid these or have more favorable pharmacokinetic and side-effect profiles. Epilepsy experts are now considering select second generation AEDs early in the treatment of the older patient with seizures, and using first generation AEDs less frequently.
Since many older patients are cared for by non-neurologists, the information regarding the new AEDs most desirable for use in older adults should be made available in a form that facilitates consideration and implementation. Indeed with clinical experience, pharmacokinetic data, study data, and fewer drug interactions, the second generation AEDs, when incorporated into the treatment regimen, can simplify treatment, maintain seizure control, and improve quality of life in this patient population. Some patients may be well-controlled on the first generation AEDs, however, second generation AEDs should now be considered early in the treatment algorithms for older patients with existing or newly diagnosed seizure disorders.
Acknowledgments: The authors are grateful to the following individuals (medical directors and consultant pharmacists in the long-term care facilities) who provided helpful comments, direction, and review of this paper: Manju Beier, PharmD, Ross Brickely, RPh, MBA, CGP, Paul Cass, MD, Mario Cornacchione, DO, CMD, Diane Crutchfield, PharmD, Sheila Deymann, PharmD, Marie Gardner, PharmD, Rob Goodwin, PharmD.
References
(1.) Cloyd JC, Lackner TE, Leppik IE. Antiepileptics in the elderly. Pharmacoepidemiology and pharmacokinetics. Arch Fam Med 1994; 3(7):589-98.
(2.) Lackner TE, Cloyd JC, Thomas LW, Leppik IE. Antiepileptic drug use in nursing home residents: Effect of age, gender, and comedication on patterns of use. Epilepsia 1998; 39(10):1083-7.
(3.) Garrard J, Harms S, Hardie N, et al. Antiepileptic drug use in nursing home admissions. Ann Nuerol 2003; 54(1):75-85.
(4.) Birnbaum A, Hardie NA, Leppik IE, et al. Variability of total phenytoin serum concentrations within elderly nursing home residents. Neurology 2003; 60(4):555-9.
(5.) Pryor FM, Ramsay RE, Rowan AJ, et al. DVA-CSP #428. Study Group. Epilepsy in Older Adults Update. Update from VA Cooperative Study #428. Epilepsia 2002; 43(Suppl 7):165-6.
(6.) Morrell MJ, Leppik I, French J, Ferrendelli J, Han J, Magnus L. The KEEPER trial: Levetiracetam adjunctive treatment of partial-onset seizures in an open-label community-based study. Epilepsy Res 2003; 54(2-3):153-61.
(7.) Morrell MJ, McLean MJ, Willmore LJ, et al. Efficacy of gabapentin as adjunctive therapy in a large, multicenter study. The Steps Study Group. Seizure 2000; 9(4):241-8.
(8.) Hauser WA. Seizure disorders: The changes with age. Epilepsia 1992; 33(Suppl 4):S6-14.
(9.) Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993; 34(3):453-68.
(10.) Cloyd JC, Remmel RP. Antiepileptic drug pharmacokinetics and interactions: Impact on the treatment of epilepsy. Pharmacotherapy 2000; 20(8 Pt 2):139S-151S.
(11.) Firth M, Prather CM. Gastrointestinal motility problems in the elderly patient. Gastroenterology 2002;122(6):1688-700.
(12.) Brodie MJ, Overstall PW, Giorgi L. Multicentre, double-blind, randomised comparison between lamotrigine and carbamazepine in elderly patients with newly diagnosed epilepsy. The UK Lamotrigine Elderly Study Group. Epilepsy Res 1999; 37(1):81-7.
(13.) Welty TE, Gidal BE, Ficker DM, Privitera MD. Levetiracetam: A different approach to the pharmacotherapy of epilepsy. Ann Pharmacother 2002; 36(2):296-304.
(14.) McLean MJ, Gidal BE. Gabapentin dosing in the treatment of epilepsy. Clin Ther 2003; 25(5):1382-406.
(15.) Werz MA, Lang P, Rienzo T. Levetiracetam therapy for epilepsy: Use and tolerability in the elderly. Epilepsia 2003;44(suppl 9):280.
(16.) Hachad H, Ragueneau-Majlessi I, Levy RH. New antiepileptic drugs: Review on drug interactions. Ther Drug Monit 2002; 24(1):91-103.
(17.) Vestergaard P, Tigaran S, Rejnmark L, Tigaran C, Dam M, Mosekilde L. Fracture risk is increased in epilepsy. Acta Neurol Scand 1999; 99(5):269-75.
(18.) Mattson RH, Cramer JA, Collins JF. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. The Department of Veterans Affairs Epilepsy Cooperative Study No. 264 Group. N Engl J Med 1992; 327(11):765-71.
(19.) Leppik IE, Wolff D. Drug interactions in the elderly with epilepsy. In: Ramsay RE, Rowan AJ. Seizures and epilepsy in the elderly. Boston: Butterworth-Heinemann, 1998: 291-302.
(20.) Sachdeo RC, Wasserstein A, Mesenbrink PJ, D'Souza J. Effects of oxcarbazepine on sodium concentration and water handling. Ann Neurol 2002; 51(5):613-20.
(21.) Martin R, Meador K, Turrentine L, et al. Comparative cognitive effects of carbamazepine and gabapentin in health senior adults. Epilepsia 2001; 42(6):764-71.
(22.) Cramer JA, Leppik IE, Rue KD, Edrich P, Kramer G. Tolerability of levetiracetam in elderly patients with CNS disorders. Epilepsy Res 2003; 56(2-3):135-45.
Author disclosures:
Dr. Leppik discloses that he has received honoraria and or/consulting fees and/or speaker's bureau fees and/or research grants from: Abbott Laboratories; Elan Pharmaceuticals; GlaxoSmithKline; MedPointe; Medtronic; Novartis; Ortho McNeil; Pfizer; Shire; UCB Pharma Inc; and Xcel Pharmaceuticals.
Dr. Bergey discloses that he has received honoraria and or/consulting fees and/or speaker's bureau fees and/or research grants from: Abbott; Cybergenics; Elan Pharmaceuticals; GlaxoSmithKline; Neuropace; Novartis; Ortho-McNeil; Pfizer; UCB Pharma, Inc.
Dr. Ramsay discloses that he has received honoraria and or/consulting fees and/or speaker's bureau fees and/or research grants from: Abbott Laboratories; Bertek; Carter Wallace; Cephalon; Cyberonics; Dainippon; Elan Pharmaceuticals; GlaxoSmithKline; IVAX; Marion Merrell Dow; Novartis; Ortho-McNeil; Pfizer; RW Johnson; Smithkline Beecham; UCB Pharma, Inc.; X-cel Pharma; he holds stock in Elan Pharmaceuticals and Pfizer.
Dr. Birnbaum discloses that she is on the speaker's bureau for Cephalon.
Dr. Elliott discloses that she has received research funding from Merck & Co., Inc.
Dr. Gidal discloses that he has received honoraria and or/consulting fees and/or speaker's bureau fees and/or research grants from: GlaxoSmithKline; Ivax; UCB Pharma, Inc.
Dr. Leppik is professor of pharmacy and adjunct professor of neurology, University of Minnesota, Minneapolis, and director of research, MINCEP Epilepsy Care, Minneapolis, Minnesota.
Dr. Bergey is professor of neurology and director, Johns Hopkins Epilepsy Center, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Dr. Ramsay is professor of neurology and psychiatry, and director, International Center for Epilepsy, University of Miami, Florida.
Dr. Rowan is professor of neurology, Mount Sinai School of Medicine, New York.
Dr. Gidal is professor of pharmacy and neurology, Pharmacy Practice Division, University of Wisconsin-Madison School of Pharmacy.
Dr. Birnbaum is assistant professor of experimental and clinical pharmacology, University of Minnesota Epilepsy Research and Education Program, Minneapolis.
Dr. Elliott is associate professor, pharmacy practice division, University of Wisconsin-Madison School of Pharmacy.
Disclosure: UCB Pharma, Inc. provided a grant for the Advisory Panel on Epilepsy in the Elderly. See last page of article for individual author disclosures.
COPYRIGHT 2004 Advanstar Communications, Inc.
COPYRIGHT 2005 Gale Group