Business Editors
PITTSBURGH--(BUSINESS WIRE)--June 11, 2002
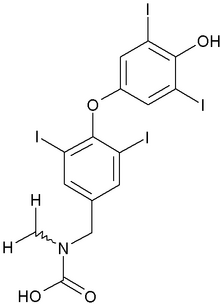
Mylan Laboratories Inc. announced today that the U.S. Food and Drug Administration has approved its Abbreviated New Drug Application (ANDA) for Levothyroxine Sodium Tablets USP, 0.025 mg, 0.05 mg, 0.075 mg, 0.088 mg, 0.1 mg, 0.112 mg, 0.125 mg, 0.15 mg, 0.175 mg, 0.2 mg and 0.3 mg.
Levothyroxine is the generic equivalent of Jerome Stevens Pharmaceuticals', Inc. Unithroid(R) Tablets, indicated for hypothyroidism and the suppression of thyroid stimulating hormones.
Mylan Laboratories Inc., is a leading pharmaceutical company that develops, manufacturers and markets generic and proprietary prescription pharmaceutical products. The company markets an extensive line of generic products through three business units, Mylan Pharmaceuticals Inc., Mylan Technologies Inc., and UDL Laboratories, Inc. and branded products through Bertek Pharmaceuticals Inc. For more information, visit www.mylan.com
To the extent any statements made in this release contain information that is not historical, these statements are essentially forward-looking statements regarding our anticipated financial results and estimates, business prospects and products in research and under going development, all of which involve substantial risks and uncertainties. Such risks and uncertainties are not predictable or quantifiable; consequently, should known or unknown risks or uncertainties materialize, or should our assumptions or estimates prove inaccurate, actual results could differ materially from those expressed or implied by such forward-looking statement. For further details and a discussion of such risks and uncertainties, we encourage you to read Forward-looking Statements found in our Annual Report on Form 10-K for the fiscal year ended March 31, 2001, and in our periodic reports on Forms 10-Q and 8-K (if any).
We assume no obligation to update any forward-looking statements presented here today, whether as a result of new information, future events or otherwise.
COPYRIGHT 2002 Business Wire
COPYRIGHT 2002 Gale Group