There has been growing international concern that mercury pollution has become a global problem--and some have suggested that global action is necessary to address it. This February, ministers and other government representatives from countries around the world will address the question of mercury pollution for a second time, at a meeting of the Governing Council of the United Nations Environment Programme (UNEP). At a previous meeting in 2003, the United States and a few other countries actively lobbied against proposals for an international convention on mercury. A global mercury program was established at that meeting; the issue of a possible international convention will be addressed again this February. How governments will answer this time depends on a combination of scientific understanding and political will.
[ILLUSTRATION OMITTED]
The political and scientific aspects of the mercury problem are complex and interrelated. Despite more than 40 years of political attention to mercury, the issue persists and is now moving back toward center stage. What is clearer today than 40 years ago is that national action alone cannot tackle the problem. New insights into developmental effects at lower exposure levels and models of atmospheric chemistry have shown that continuing emissions and their global transport still pose environmental and human health risks. Mercury pollution--which has sources all over the world--affects all countries as well as global commons such as the oceans. In addition, mercury's presence in international commerce makes single-nation efforts insufficient to address the problem effectively.
The problem of mercury in the environment involves numerous aspects, ranging from its emissions and transport through the biosphere all the way to its accumulation in and toxicity to living organisms. Potential policy actions on mercury will build heavily upon the current "state of the science" on mercury pollution--particularly its potential for long-range transport--and on the threat posed to human health and development. Future policies should also build upon the numerous actions already taken to address mercury--domestically and internationally--and should involve industrialized as well as developing nations.
Mercury in the Environment
Mercury is an element in the periodic table--its symbol is Hg. As an element, mercury has always been present in the Earth's environment and will always be there. However, human activities have dramatically changed where mercury is found in the Earth as well as the forms it takes in the environment.
Mercury has been known to human civilizations for millennia and has been used as a pharmaceutical, in religious rites, and in mining. (1) Its toxicity has been recognized as well since as early as the first century. (2) Exposure to mercury can cause, among other symptoms, neurological problems and reproductive and developmental abnormalities.
[FIGURE 1A OMITTED]
[FIGURE 1B OMITTED]
Over centuries, human activities have removed mercury from deep reservoirs in the Earth and mobilized it in the biosphere, creating vast disruptions in the natural cycle of the element. Figures 1a and 1b on page 24 show the biogeochemical cycling of mercury in the preindustrial era compared to the current cycle. As is shown in Figure 1b, mercury mobilized by humans has accumulated in the atmosphere, soils, and oceans. The only sink for mercury is deep ocean burial, which happens extremely slowly. This means that all the mercury that has historically been released by human activities, as well as the mercury currently emitted, will take hundreds if not thousands of years to return to deep reservoirs in the Earth.
Mercury's Forms
Mercury exists in many forms. It may be most familiar as the dense, silvery liquid used, among other places, in old thermometers. It is still used in some consumer products, such as fluorescent light bulbs and thermostats. More recently, mercury's industrial uses have included cement and iron and steel production. Mercury contained in products can be released into the atmosphere upon the products' disposal. Mercury is also present in fossil fuels such as coal and is emitted as a byproduct of coal burning; such combustion is currently the largest anthropogenic source.
In the air, mercury can take many different forms. Most commonly, it exists in the atmosphere in its elemental (ground) state as a gas, referred to as Hg(0). Through reactions in the atmosphere, it can oxidize to a positively charged form referred to as Hg(II) or divalent mercury. Hg(II) is very soluble in the atmosphere, so it can be dissolved in atmospheric water and rained out of the atmosphere; this is the predominant form of mercury that enters terrestrial and marine ecosystems through deposition. Mercury can also be associated with atmospheric particulate matter; this is termed Hg(P). (3) Measurements of mercury use slightly different terminology--they report data for Hg(0); Hg(P); total gaseous mercury (TGM), comprising all mercury in the gas phase; and reactive gaseous mercury (RGM), which is Hg(II) in the gas phase. (4) (The box below provides further detail on forms of mercury in the environment.)
When examining the environmental impacts of mercury, another form is extremely important: methyl mercury. Though it makes up only a small portion of the total mercury in the environment, it is the most significant form that results in toxic environmental exposures to humans. Methyl mercury is formed by bacteria that take inorganic mercury and convert it to an organic form. This organic form is taken up by marine organisms, and it bioaccumulates through food webs. (5) Methyl mercury thus poses a risk to human health and the environment. High levels of mercury in fish such as tuna have prompted consumption advisories, particularly for pregnant women and children, in a number of countries and several U.S. states. (6)
Since the beginning of the industrial era, the concentrations of mercury in the atmosphere have increased threefold. (7) Significant industrial sources of mercury include coal-fired power plants and waste incineration. (8) Emissions have recently decreased in Europe and North America, but they continue to be regionally significant. Asian emissions have been increasing rapidly and now account for 50 percent of global emissions (Table 1 on page 26 provides a breakdown of emissions by region for 1995, the latest estimates available from the Global Emissions Inventory Activity). (9)
Human and Environmental Toxicity
Though research on mercury's atmospheric behavior is ongoing, it is well-known that mercury travels a great distance through the air, and its effects can be seen far from regions where it is emitted. For example, high levels of mercury have been found in the Arctic ecosystem. Due to accumulation of methyl mercury there, Arctic indigenous peoples can be particularly affected by such pollution through consumption of traditional foods: A recent Arctic Monitoring and Assessment Programme (AMAP) report concluded that there is evidence that current mercury exposures pose a health risk to some humans and animals in the Arctic. (10)
In the modern era, one of the first major incidents that raised mercury as a serious environmental issue was a poisoning incident in Minamata, Japan, in the 1950s. Methyl mercury, emitted by a factory to local waters, contaminated fish, and those who consumed large amounts of contaminated fish became ill. Symptoms of Minamata disease included neurological damage and disturbances of sensation and movement. (11) In the early 1970s, people in areas of rural Iraq contracted mercury poisoning after eating seeds treated with mercury-based pesticides. (12) These exposures caused effects not only in those directly exposed but also in children exposed in utero.
More recently, low-dose exposures of methyl mercury have also been linked to health effects. Three major studies have been conducted on neurological and developmental effects of mercury exposure in pregnant women and children. These studies took place in the Faeroe Islands, Seychelles, and New Zealand. (13) Of these three studies, the Seychelles study found no effect linked to mercury exposure, and the Faeroe Islands and New Zealand studies did find adverse effects. In particular, in the Faeroe Islands study, which looked at pregnant women exposed to mercury through eating pilot whale meat, the researchers found significant associations between mercury exposure and effects on language, attention, and memory. The U.S. Environmental Protection Agency (EPA) chose to use the Faeroe Islands study as the basis for their reference dose (RfD)--which is an estimate of daily exposure that would result in no increased risk. (14)
Scale of the Mercury Problem
There is a growing realization that mercury's impacts are global. A recent modeling study calculated that anthropogenic emissions from outside North America contribute 37 percent to total mercury deposition in the contiguous United States, while North American anthropogenic emissions contribute 30 percent. (15) Models of mercury's behavior in the atmosphere exist and can help to illuminate mercury's behavior in the environment. The box on page 27 provides more information on mercury modeling.
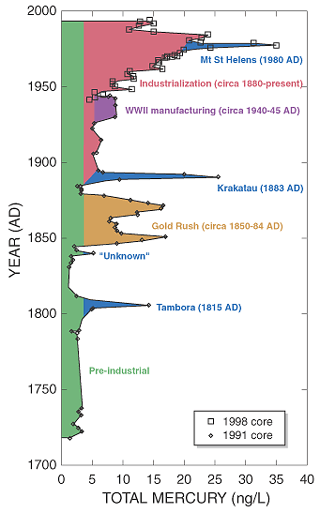
Although mercury clearly reaches locations far from its initial release, it can also be a local issue. The Commission for Environmental Cooperation identified 244 "hot spots" of mercury contamination in North America, where levels were measured to be higher than the background level (that is, the baseline level that is present everywhere in the environment). (16) Several of these sites were located in the vicinity of major sources, such as waste incinerators or former mining operations. For example, miners during the California Gold Rush of the 1840s and 1850s used mercury-based processes to extract gold. Contamination is still present even 150 years later, and the state of California has issued pollution advisories for fish in impacted lakes. (17)
[ILLUSTRATION OMITTED]
Scientific Questions
Mercury's behavior in the atmosphere and biosphere is the subject of much current scientific research. Until recently, it was thought that mercury was very long-lived in the atmosphere, taking about a year to circle the globe as Hg(0) before converting to Hg(II) and raining down to earth. Recent research has questioned these assumptions and has begun to raise interesting scientific questions about how mercury behaves in the environment. For example, measurements taken in the Arctic have revealed a curious phenomenon: Shortly after sunrise, in Arctic spring, levels of Hg(0) have been observed to rapidly decline concurrently with spikes in levels of RGM. (18) This occurs several times over a period of weeks and has been observed in several different Arctic locations as well as in the Antarctic. (19) These Arctic measurements show that mercury's behavior in the environment is more dynamic than previously thought. Similar reactions may be occurring in the marine boundary layer, the lowest part of the atmosphere above the oceans, according to a recent study in the Mediterranean. (20)
Another area of scientific interest is the estimation of natural sources. While it is clear that human activity has dramatically influenced the mercury cycle, there are many uncertainties surrounding how mercury mobilizes from its natural sinks. For example, recent research measuring mercury's evasion from naturally enriched areas has revealed that previous estimates of natural mercury emission from land were likely too low. (21) In addition, the remobilization of previously deposited mercury is not yet well understood. Current studies are examining the pathways by which mercury mobilizes from ecosystems and into the biosphere.
A study called METAALICUS (Mercury Experiment to Assess Atmospheric Loading in Canada and the United States) has explored the effect of changes in mercury atmospheric deposition on concentrations in fish. (22) This study, which uses stable isotopes of mercury to distinguish mercury added to the ecosystem from native mercury, aims to illuminate the differences in environmental behavior between newly emitted mercury and the legacy mercury, which remains in the environment from previous contamination and natural sources. Results from this study have shown that "new" mercury is more likely to be methylated and accumulate in fish than "old" mercury. (23) Further research is ongoing.
The study's finding has important implications for policy--it means that cuts in current emissions of mercury could lead to a more rapid decline in methyl mercury concentrations in fish, and therefore potential toxic exposures, than previously thought.
Controlling Mercury
A number of domestic and international policy actions have addressed mercury in the environment. The United States regulates mercury emissions from municipal waste combustion, medical waste incineration, and chlor-alkali production; several U.S. states have regulated mercury. Current proposals to regulate sources of mercury from coal-fired power plants are generating controversy. There are also proposals aiming to ban the use of mercury in consumer products. In addition, the United States has participated in two regional international agreements that have addressed mercury. Table 2 on pages 30 and 31 provides a timeline of policy actions on mercury.
Mercury Regulation in the United States
Because mercury is a multimedia pollutant and is present in many forms in the environment, it is regulated in a variety of different ways by different authorities.
EPA has regulated mercury emissions from two major sources: municipal waste combustors and medical waste incinerators. Regulations on these sources were issued in 1995 and 1997, respectively. However, EPA does not yet regulate the most significant source of mercury emissions, coal-fired power plants. Some states, such as Massachusetts, already regulate mercury from such plants. (24)
The regulations on mercury emissions have made a significant impact in the United States. Figure 2 on page 32 shows emissions of anthropogenic mercury in 1990, 1996, and 1999. As shown, the total emissions of mercury went from 220 tons to 120 tons, a 45 percent decrease over that 9-year period. This was mostly due to substantial cuts in emissions from medical waste incinerators and municipal waste combustors, the two regulated sources. However, mercury from "utility coal boilers," or coal-fired power plants remained unchanged and in 1999 was the most significant single source of anthropogenic mercury emission.
In December 2000, EPA, under legal pressure from environmental groups, decided to regulate mercury from power plants under the Clean Air Act. These regulations, which were to be proposed by December 2003, would have required mercury to be regulated based on the "maximum achievable control technology" standard. (25) However, in 2003, the Bush administration proposed to reverse this determination, as part of its "Clear Skies" initiative, arguing that mercury should be controlled using a cap-and-trade mechanism similar to that used to control acid rain. (26) The new proposed regulations on mercury have drawn significant criticism from environmental groups. The Sierra Club has noted that the Bush administration plan would allow 520 percent more mercury pollution by 2010 than would existing protections under the Clean Air Act. (27) The electric power industry, on the other hand, is strongly in favor of the "Clear Skies" initiative. (28)
Opposition to further controls on mercury, especially from coal-fired power plants, has focused on issues of cost and feasibility. (29) Industrial lobbyists from coal and electric power interests argue that these regulations would be too costly and burdensome. The Bush administration has been sympathetic to their arguments and has altered regulations to facilitate their needs. However, industry arguments about unreasonable costs are countered by the experience of mercury reduction on the state level as several industrially and technically advanced states have moved forward to dramatically reduce mercury emissions in a cost-effective manner.
Several U.S. states have taken action to control mercury pollution, and in the absence of strong leadership on mercury at the federal level, these efforts have gone farther than national initiatives. For example, the governors of the New England states, in collaboration with the premiers of the eastern Canadian provinces, jointly agreed to a mercury action plan in 1998. (30) The plan sets an ultimate goal of virtually eliminating anthropogenic mercury emissions in the region. The region has already achieved its goal of a 50 percent cut in emissions by 2003; in 2001, the group set a further goal of reducing emissions by 75 percent by 2010. (31) The cuts thus far have been achieved without significant economic effects to the industries involved, and the regulations have been actively supported by Democratic and Republican governors in the region.
[ILLUSTRATION OMITTED]
In addition to addressing mercury emissions, there have also been increasing efforts in the United States to educate consumers about mercury risks, particularly the risk from consumption of seafood. More than 300,000 babies are born every year in the United States with mercury exposures that exceed recommended levels. (32) Acknowledging that levels of mercury in some fish and shellfish pose risks to vulnerable populations, the U.S. Food and Drug Administration in March 2004 issued updated dietary guidelines for pregnant women, children, and nursing mothers. (33)
The recommendations instruct these vulnerable groups to avoid eating particular species of fish high in mercury (shark, swordfish, king mackerel, and tilefish) while limiting consumption of fish lower in mercury (for example, shrimp, canned light tuna, salmon, pollock, and catfish) to 12 ounces, or two average meals, per week. For albacore (white) tuna, which is higher in mercury than light tuna, the recommendations suggest limiting consumption to 6 ounces per week. However, the recommendations note that consumption of fish has significant health benefits--among them, cardiovascular health benefits from omega-3 fatty acids--and these benefits must be taken into account while balancing potential risks caused by contamination.
International Actions on Mercury
Mercury has a long history of regulation as an international problem. Heavy metals were identified as pollutants of concern at the 1972 United Nations Conference on the Human Environment held in Stockholm. Dumping of mercury at sea was prohibited in the 1972 Oslo Convention on the Protection of the Marine Environment by Dumping from Ships and Aircraft. (34) In 1973, the Organisation for Economic Co-operation and Development (OECD) recommended that all its member countries adopt measures to reduce anthropogenic emissions of mercury to the environment to the lowest possible levels. (35)
Although this early international action on mercury reflected concern and prompted significant research efforts throughout the 1970s, mercury continued to pose problems. Several factors contributed to mercury's reemergence as a high-profile international issue in the 1990s. A growing concern about low-dose effects of pollutants, including mercury, began to emerge with new scientific information and epidemiological studies. Growing concerns about mercury's presence in the Arctic environment galvanized support for international action among affected countries and indigenous populations. In addition, a growing awareness of mercury problems emerged in developing countries, whose industrial capacities were expanding. While emissions from Europe and North America had declined significantly, emissions from Asia, particularly from China, were increasing. These increasing emissions threatened to overwhelm the progress made by cutting mercury in other regions.
On a regional basis, the United States has participated in North American actions on mercury. Under the auspices of the Commission for Environmental Cooperation's Sound Management of Chemicals initiative, the United States, Canada, and Mexico have agreed on two successive regional action plans for mercury, the latest in 2000. These action plans set specific goals and objectives for reducing mercury emissions to the environment.
The most comprehensive international agreement regulating mercury to date is the 1998 Arhus Protocol on Heavy Metals to the Convention on Long-Range Transboundary Air Pollution (LRTAP). The LRTAP convention's geographical scope covers the United States and Canada, Western and Eastern Europe, and Russia. The Arhus Protocol, which covers the heavy metals mercury, cadmium, and lead, entered into force in 2003, and currently has 21 parties, including the United States. (36) According to the protocol, parties must reduce their emissions of mercury below 1990 levels (or an alternate year between 1985 and 1995). It establishes limit values for mercury emissions from municipal and hazardous waste incineration and suggests best available techniques for limiting emissions from stationary industrial sources.
In another regional setting, the Arctic Council, which consists of the eight Arctic countries and six permanent participants representing Arctic indigenous groups, identified mercury as a high-priority pollutant in the Arctic ecosystem through its assessments of the state of the Arctic environment, including the Arctic Monitoring and Assessment Programme (AMAP). In 2000, the Arctic Council drew particular attention to mercury, urging a global assessment of mercury as a basis for future international action. The latest AMAP report in 2002 drew attention to mercury, and its work on mercury was one of the more controversial areas of assessment. It cited the high exposures to mercury in the Arctic region and noted that "reducing exposure to mercury can only be addressed by regional and global action to reduce worldwide emissions." (37)
[FIGURE 2 OMITTED]
Recognizing this growing concern and emerging regional actions on mercury, the United Nations Environment Programme's Governing Council in 2001 commissioned the Global Mercury Assessment. (38) Completed in 2002, the assessment concluded that mercury is a global pollutant with serious effects, particularly in vulnerable populations and ecosystems. It also found that policy action could have a significant effect on mercury levels in the environment but that local and regional action by itself was not sufficient to address the problem. The box on page 33 details further information on the Global Mercury Assessment process.
In 2003, the UNEP Governing Council considered the Global Mercury Assessment and concluded that there is sufficient evidence of significant global adverse impacts from mercury to warrant further international action to reduce the risks to humans and wildlife from the release of mercury to the environment. (39) It initiated a program to raise awareness of mercury and support capacity-building exercises on mercury pollution. At its next meeting, in February 2005, the Governing Council will take up the issue of whether to begin negotiating an international convention on mercury.
Technical Options
Technologies and policy options for reducing mercury emissions exist and in many cases are already in place. Technological controls exist to limit mercury emitted from industrial point sources (such as chlor-alkali production or coal-fired power plants). Other policy options include phasing out mercury use in products and collecting and treating mercury-containing wastes.
Mercury emissions have greatly declined in North America and Europe over the past two decades. One significant reason for this is the reduction of mercury that has been achieved as cobenefits when technologies are applied to reduce sulfur dioxide (S[O.sub.2]), nitrogen oxides (N[O.sub.x]), and particulate matter (PM). Controls for PM can capture Hg(P), while controls for S[O.sub.2] can capture Hg(II). (40) In the United States, as discussed above, regulations under the Clean Air Act have reduced emissions significantly from municipal waste combustors and medical waste incinerators.
The technologies for reducing mercury from coal-fired power plants depend greatly on which form of mercury is emitted from the plant. These power plants emit all three forms of mercury--Hg(0), Hg(II), and Hg(P)--but in different ratios, which are dependent on many factors, including the composition of the coal burned. While the majority of mercury emissions on the East Coast are in the oxidized Hg(II) form, West Coast emissions are mainly in the form of Hg(0). (41) Where plants emitting Hg(II) can easily take advantage of co-benefits from addressing other pollutants, those emitting mostly Hg(0) may need to apply technologies designed specifically to deal with mercury. These technologies exist; however, they have not been implemented yet on a large scale.
Scientific Uncertainty and Policy Challenges
Regulation of mercury has been and continues to be an area of much controversy. Interest groups have lobbied heavily on both sides of the issue. Some have argued that mercury must be virtually eliminated as soon as possible. (42) Others have drawn attention to scientific uncertainties, taking advantage of limited data to argue that further cuts in, for example, utility emissions, would not be cost-effective. (43)
Indeed, there are a number of open scientific questions about mercury and, in particular, its behavior in the environment. These include: How much mercury comes from "natural" as opposed to anthropogenic sources? Will reductions in anthropogenic emissions have an effect on concentrations in fish, and if so how long will this take? Many of these kinds of questions are being addressed by current research, in modeling studies as well as experimental research.
In addition to regulatory controversies posed by scientific uncertainty, a significant policy challenge in controlling mercury is that regulation affects powerful industry groups. The U.S. position and its regulations on mercury during the Bush administration can be seen as reflecting strong influence from lobbying groups in the coal industry. Historically, the electric power industry has also been a strong corporate lobby. The concerns of the industry center on cost and feasibility, and the coal industry in particular is located in key, powerful states with impacts on national elections. In addition, despite epidemiological studies, scientific assessments, and dietary guidelines to the contrary, U.S. business continues to argue that mercury exposure from fish is not harmful. (44) Environmental groups have also made mercury a priority issue and have argued strongly for further regulation. Thus, mercury has become an increasingly polarized issue.
[ILLUSTRATION OMITTED]
Internationally, the United States has thus far resisted international cooperation. In addition to the same cost concerns that prevent national action going forward, the Bush administration has thus far not been willing to engage substantively in many areas where international environmental action would require additional resources, as would be the case with an international mercury agreement. Given the high percentage of U.S. mercury deposition that comes from international sources, however, this seems a shortsighted position. To truly address domestic mercury problems, international action will be necessary. If the United States is serious about reducing levels of methyl mercury in the environment, it should engage more productively with the international community on this issue.
[ILLUSTRATION OMITTED]
In contrast to U.S. resistance, the European Union and the group of Latin American and Caribbean nations have pushed for consideration of a treaty. (45) The European Union in particular has been spurred on by its more environmentally active member states (such as Sweden, which has recently proposed laws that would ban mercury in commerce or use), (46) and it is also in the final stages of developing a strategy to deal with mercury. (47) In addition, an international group of environmental nongovernmental organizations including Greenpeace, the Natural Resources Defense Council, and the European Environmental Bureau have lobbied in favor of a global treaty.
Conclusion: Is Global Action Necessary?
Scientific research to date has established conclusively that humans have had a significant impact on the mercury cycle. Mercury pollution has risen to levels that might be expected to impact human health as well as the environment. Indeed, there are uncertainties regarding the pathways by which mercury reaches the human food chain and its eventual toxicity. However, mercury seems a clear case where application of the precautionary principle would be warranted.
Significant data exists that mercury is a persistent and growing international problem, and waiting for full scientific certainty on outstanding questions of toxicity and transport would not be a prudent course of action considering the potential harm to humans and the environment. In fact, UNEP's Global Mercury Assessment concluded that "[d]espite data gaps, sufficient understanding has been developed of mercury (including knowledge of its fate and transport, health and environmental impacts, and the role of human activity), based on extensive research over half a century, that international actions to address the global mercury problem should not be delayed." (48)
Mercury is clearly a worsening global problem, and dealing with it will require concerted action on the part of multiple countries and regions. This is consistent with the conclusions of the Global Mercury Assessment, which confirmed the need for a global approach to address the problem of mercury. The assessment identified four major reasons why local and regional action would not be sufficient: global cycling of mercury, which worsens mercury contamination due to global background levels; impacts on global fishing and fish stocks; mercury's disproportionate burden in less-developed regions; and the presence of mercury in international trade and commerce. (49)
An international, legally binding convention on mercury would be one approach to this type of global problem. Negotiators of a global convention would have to consider issues of technological feasibility as well as equity and financing. Addressing mercury would have obvious benefits--both domestically and internationally. Because mercury is a local as well as global problem, developing countries in particular could take advantage of financial and technical assistance at the global level to address problems that impact the local and the global environment. In this way, negotiations on mercury could proceed similarly to the successful negotiations of the Stockholm Convention on Persistent Organic Pollutants, during which countries were able to identify a dual benefit of reducing global pollution while dealing with local problems such as managing unwanted chemical stockpiles and strengthening regulatory infrastructure. (50) One advantage of an international convention would be to focus political attention and resources on the mercury problem, setting a framework within which donor governments and international organizations could provide assistance aimed at fulfilling defined reduction goals.
However, significant progress could be made whether or not negotiations begin on a global convention: through bilateral and multilateral action focusing on capacity building and technology transfer for reducing emissions, especially in areas where emissions currently are rising. In particular, such actions could address the large and growing Asian sources, particularly in China. Asia emits 50 percent of the world's anthropogenic mercury and also has significant local air pollution problems from S[O.sub.2] and N[O.sub.x] emissions. Collaborative efforts to deal with Asian air pollution problems could help to build technological capacity in these industrializing economies and might be implemented in partnership with the World Bank or other international organizations. (51) Such efforts could take advantage of significant co-benefits to reduce mercury emissions as well. This action will have to overcome significant opposition from industrial groups, however, which have argued that emissions controls are too costly and infeasible.
Ultimately, a satisfactory solution to the mercury problem will require concerted efforts on the part of all nations and will have to go beyond first steps that build upon co-benefits. This will require a combination of resources and political will. Governments have a unique opportunity at the upcoming UNEP Governing Council meeting in February 2005 to continue to build a process to address the mercury contamination problem worldwide. The health, development, and well-being of future generations worldwide will depend on their choice of action.
NOTES
1. J. O. Nriagu, "Production and Uses of Mercury," in J. O. Nriagu, ed., The Biogeochemistry of Mercury in the Environment (Amsterdam: Elsevier, 1979), 23-40.
2. W. H. Schroeder and J. Munthe, "Atmospheric Mercury--an Overview," Atmospheric Environment 32, no. 5 (1998): 809-22.
3. United Nations Environment Programme (UNEP), Global Mercury Assessment, (Geneva, 2002). http://www.chem.unep.ch/mercury.
4. R. Ebinghaus et al., "International Field Intercomparison Measurements of Atmospheric Mercury Species at Mace Head, Ireland," Atmospheric Environment 33 (1999): 3063-73.
5. UNEP, note 3 above.
6. U.S. Environmental Protection Agency (EPA), Fish Advisories, http://www.epa.gov/ost/fish/ (accessed 9 September 2004).
7. R. P. Mason and G. R. Sheu, "Role of the Ocean in the Global Mercury Cycle," Global Biogeochemical Cycles 16, no. 4 (2002): 1093.
8. E. G. Pacyna and J. M. Pacyna, "Global Emission of Mercury from Anthropogenic Sources in 1995," Water, Air & Soil Pollution 137 (2002): 149-65.
9. Ibid.
10. Arctic Monitoring and Assessment Programme (AMAP), AMAP Assessment 2002: Human Health in the Arctic (Oslo, 2003), http://www.amap.no.
11. Ministry of the Environment Japan, National Institute for Minamata Disease, http://www.nimd.go.jp/english/index.html (accessed 31 August 2004).
12. UNEP, note 3 above.
13. P. Grandjean et al., "Cognitive Deficit in 7-Year-Old Children with Prenatal Exposure to Methylmercury," Neurotoxicology and Teratology 19, no. 6 (1997): 417-28; K. S. Crump et al., "Influence of Prenatal Mercury Exposure Upon Scholastic and Psychological Test Performance: Benchmark Analysis of a New Zealand Cohort," Risk Analysis 18, no. 6 (1998); and G. J. Myers et al., "Prenatal Methylmercury Exposure from Ocean Fish Consumption in the Seychelles Child Development Study." Lancet 361 (2003): 1686-92.
14. EPA, Water Quality Criterion for the Protection of Human Health: Methylmercury, EPA-823-R-01-001 (Washington, DC, 2001), http://www.epa.gov/waterscience/criteria/methylmercury/merctitl.pdf.
15. C. Seigneur et al., "Global Source Attribution for Mercury Deposition in the United States," Environmental Science & Technology, 15 January 2004, 555-69.
16. Commission for Environmental Cooperation, Mercury Hot Spots of North America, http://www.cec.org/files/PDF/POLLUTANTS/hotspots_en.pdf (accessed 9 September 2004).
17. Ibid.
18. W. H. Schroeder et al., "Arctic Springtime Depletion of Mercury," Nature, 23 July 1998, 331-32.
19. AMAP, Arctic Pollution 2002 (Oslo, 2002) (for a review of this report, see also N. Eckley and H. Selin, "The Arctic at Risk," Environment, September 2003, 37-40); T. Berg et al., "Arctic Mercury Depletion Events at Two Elevations as Observed at the Zeppelin Station and Dirigibile Italia, Ny-Alesund, Spring 2002," Journal De Physique IV 107 (2003); 151-54: R. Ebinghaus et al., "Antarctic Springtime Depletion of Atmospheric Mercury," Environmental Science & Technology, 15 March 2002, 1238-44.
20. I. M. Hedgecock, N. Pirrone, F. Sprovieri, and E. Pesenti, "Reactive Gaseous Mercury in the Marine Boundary Layer: Modelling and Experimental Evidence of Its Formation in the Mediterranean Region," Atmospheric Environment 37 (2003): S41-S49.
21. M. A. Engle and M. S. Gustin, "Scaling of Atmospheric Mercury Emissions from Three Naturally Enriched Areas: Flowery Peak, Nevada; Peavine Peak, Nevada; and Long Valley Caldera, California," The Science of the Total Environment 290 (2002): 91-104.
22. University of Alberta, Metaalicus, http://www.biology.ualberta.ca/metaalicus/metaalicus.htm (accessed September 9 2004).
23. C. L. Babiarz et al., "A Hypolimnetic Mass Balance of Mercury from a Dimictic Lake; Results from the Metaalicus Project," Journal De Physique IV 107, no. 83-86, Part 1 (2003).
24. Commonwealth of Massachusetts, "Massachusetts Emissions Standards for Power Plants," in 310 CMR 7.29 (2004).
25. "Regulatory Finding on the Emissions of Hazardous Air Pollutants from Electric Utility Steam Generating Units," Federal Register 65, no. 245 (2000): 79825-31.
26. "Proposed National Emission Standards for Hazardous Air Pollutants; and, in the Alternative, Proposed Standards of Performance for New and Existing Stationary Sources: Electric Utility Steam Generating Units; Proposed Rule," Federal Register 69, no. 20 (2004): 4652-752.
27. Sierra Club, "Clear Skies" = Bush Smokescreen, http://www.sierraclub.org/cleanair/clear_skies.asp (accessed September 9 2004).
28. Edison Electric Institute, Clear Skies Act of 2003, http://www.eei.org/industry_issues/environment/air/multi-emissions/Clear_Skies_Act_of_2003/index.htm (accessed September 9 2004).
29. See, for example, the response by the World Coal Institute to the European Commission's stakeholder consultation on mercury, http://europa.eu.int/comm/environment/chemicals/mercury/pdf/world_coal_institute.pdf.
30. New England Governors and Eastern Canadian Premiers, Mercury Action Plan, (Frederickton, New Brunswick: The Committee on the Environment of The Conference of New England Governors and Eastern Canadian Premiers, 1998), http://www.mass.gov/dep/ors/files/negecp.pdf.
31. UNEP, note 3 above.
32. Commission for Environmental Cooperation, note 16 above.
33. U.S. Department of Health and Human Services and EPA, What You Need to Know About Mercury in Fish and Shellfish: 2004 EPA and FDA Advice For: Women Who Might Become Pregnant, Women Who Are Pregnant, Nursing Mothers, Young Children, EPA-823-R-04-005 (Washington, DC, 2004). http://www.cfsan.fda.gov/~dms/admehg3.html.
34. UNEP, note 3 above.
35. Organisation for Economic Co-operation and Development (OECD), Recommendation of the Council on Measures to Reduce All Man-Made Emissions of Mercury to the Environment, C(73)172/Final (Paris: OECD, 1973), information is accessible via http://webdominol.oecd.org/horizontal/oecdacts.nsf/Display/4EE1D8AE4845942CC1256F01005BE780? OpenDocument.
36. For updated status of ratifications, see United Nations Economic Commission for Europe, Status: POPs Protocol, http://www.unece.org/env/Irtap/status/98hm_st.htm (accessed 9 September 2004).
37. AMAP, note 19 above, p. ix.
38. UNEP, note 3 above.
39. UNEP, Chemicals: Mercury Programme, Decision 22/4.7 February 2003, http://www.chem.unep.ch/mercury/mandate-2003.htm (accessed 8 November 2004).
40. J. H. Pavlish et al., "State Review of Mercury Control Options for Coal-Fired Power Plants," Fuel Processing Technology 82, no. 2-3 (2003): 89-165.
41. Ibid.
42. Clean Water Action, New England Zero Mercury Campaign, http://www.cleanwateraction.org/mercury/(accessed 9 September 2004).
43. R. Lutter and E. Irwin, "Mercury in the Environment: A Volatile Problem," Environment 44, no. 9 (2002): 24-40.
44. U.S. Chamber of Commerce, U.S. Chamber Position on Mercury Emissions, http://www.uschamber.com/government/issues/environment/mercuryemissions.htm (accessed 13 September 2004).
45. United Nations Environment Programme (UNEP), Report of the Global Mercury Assessment Working Group on the Work of Its First Meeting, UNEP(DTIE)/GMA/WG.1/8 (Geneva: UNEP, 2002).
46. Kemikalieinspektionen (Swedish Chemicals Inspectorate), Kvicksilver--Utredning Om Ett Generellt Nationellt Forbud (Mercury--Study on a General National Ban). (Stockholm: KEMI, 2004), http://www.kemi.se/upload/Trycksaker/Pdf/Rapporter/Rapport2_04.pdf.
47. European Commission, Development of an EU Strategy on Mercury, http://europa.eu.int/comm/environment/chemicals/mercury/ (accessed 9 September 2004).
48. UNEP, note 3 above.
49. Ibid.
50. N. Eckley, "Traveling Toxics: The Science, Policy, and Management of Persistent Organic Pollutants," Environment, September 2001, 24-36.
51. H. Selin, "Comment on "Intercontinental Transport of Air Pollution: Will Emerging Science Lead to a New Hemispheric Treaty?"" Environmental Science & Technology, 15 March 2004, 1912-13.
RELATED ARTICLE: FORMS OF MERCURY IN THE ENVIRONMENT
In the environment, mercury exists in a number of different forms, each of which have different properties and toxicities. The following is a summary of key forms of mercury in the environment and an introduction to common mercury measurements in the atmosphere.
Chemical Forms
* Elemental, or metallic mercury (Hg(0)), is the pure form of mercury. Hg(0) is the silvery liquid most commonly associated with thermometers; however, it can also exist as a gas, which is colorless and odorless.
* Mercury can form complexes with other compounds, most frequently as divalent mercury, which can be written as Hg(II) or H[g.sup.2+]. Such compounds include mercuric chloride (Hg[Cl.sub.2]), mercuric oxide (HgO), and mercuric sulfide (HgS).
* Methyl mercury is the most common organic mercury form in the environment (other forms of organic mercury include dimethylmercury, phenylmercury, and ethylmercury). It is formed in the environment predominantly through microbes that methylate mercury, but can also be formed by abiotic (chemical) processes. Methyl mercury is the predominant form of mercury in fish. Because of this and because it is particularly toxic, it is the most significant mercury form of concern with respect to toxicity and human exposure.
Mercury Measurements in the Atmosphere
* Total gaseous mercury (TGM) is a measure of all mercury that exists as a gas in the atmosphere. This is made up of mostly Hg(0) but also includes a small contribution from Hg(II).
* Reactive gaseous mercury (RGM) includes all Hg(II) that exists in the gas phase.
* Total particulate mercury (TPM) is a measure of all mercury bound to atmospheric particulate matter.
RELATED ARTICLE: MODELING MERCURY
Models of mercury in the atmosphere can illuminate where mercury has come from, what transformations it undergoes in the atmosphere, and where it ultimately deposits. Atmospheric models of mercury exist on global, regional, and local scales. (1) Current models reproduce well the general patterns of total gaseous mercury in the atmosphere, but further work is needed to better constrain the rates of reactions and transformations in the atmosphere. (2) One of the more recent, currently under development, is a mercury model using the GEOS-CHEM global tropospheric chemistry and transport model. (3) The GEOS-CHEM mercury model incorporates the emission of mercury (as Hg(0), Hg(II), and Hg(P)), as well as transformations mercury undergoes in the atmosphere (such as Hg(0) oxidizing to form Hg(II)). It also allows for dynamic simulations of mercury deposition and re-emission, so mercury's transport can be tracked not only through the atmosphere but through the biosphere and oceans as well. These simulations will help scientists and policymakers understand mercury's complex behavior in the global environment. The two figures to the right show initial results from the GEOS-CHEM mercury simulation for global average total gaseous mercury (TGM) and mercury deposition over the United States.
[ILLUSTRATION OMITTED]
[ILLUSTRATION OMITTED]
1. P. Pai, P. Karamchandani, and C. Seigneur, "Simulation of the Regional Atmospheric Transport and Fate of Mercury Using a Comprehensive Eulerian Model," Atmospheric Environment 31, no. 17 (1997): 2717-32; O. R. Bullock Jr. and K. A. Brehme, "Atmospheric Mercury Simulation Using the CMAQ Model: Formulation Description and Analysis of Wet Deposition Results," Atmospheric Environment 36, no. 2135-46 (2002).
2. A. Ryaboshapko et al., "Comparison of Mercury Chemistry Models," Atmospheric Environment 36, no. 24 (2002): 3881-98.
3. More on the GEOS-CHEM model in general can be found at http://www-as.harvard.edu/chemistry/trop/geos/index.html; see also I. Bey et al., "Global Modeling of Tropospheric Chemistry with Assimilated Meteorology: Model Description and Evaluation," Journal of Geophysical Research 106, no. D19 (2001): 23073-095. The GEOS-CHEM mercury simulation is currently under development by Noelle Eckley Selin, Rokjin J. Park, and Daniel J. Jacob (Harvard University), in collaboration with Sarah Strode and Lyatt Jaegle (University of Washington).
RELATED ARTICLE: UNEP'S GLOBAL MERCURY ASSESSMENT
The Global Mercury Assessment is the latest, most comprehensive policy-focused assessment of mercury pollution at global scale. (1) The United Nations Environment Programme (UNEP) began the Global Mercury Assessment in 2001, asking governments, intergovernmental and nongovernmental organizations, and the private sector to submit information on mercury. Submissions were received from 81 governments, 10 intergovernmental organizations, and 12 nongovernmental organizations. The assessment process was guided by a working group consisting of experts nominated by governments and international and nongovernmental organizations. The assessment's mandate covered eight different topics, including chemistry, sources, control technologies, national initiatives, policy options, and an analysis of data gaps. The assessment concluded that mercury is present throughout the global environment, cycles globally, and has serious effects on human health and the environment. It also found that the mercury problem could be addressed by concerted policy action at the international level.
As a follow-up to the Global Mercury Assessment, UNEP has organized a series of awareness-raising workshops on mercury in developing countries to prepare these countries to deal with the mercury problem at national, regional, and global scales. Workshops have been held in Thailand (April 2004), South Africa (June 2004), and Ukraine (July 2004), among others. Ongoing activities aim to facilitate capacity building and technical assistance for countries' efforts, especially in developing countries, to tackle mercury pollution issues.
1. United Nations Environment Programme (UNEP), Global Mercury Assessment, (Geneva: UNEP, 2002), http://www.chem.unep.ch/mercury.
Noelle Eckley Selin is a PhD candidate in the Department of Earth and Planetary Sciences, Harvard University, where she models the global chemistry and transport of mercury. She has published articles and book chapters on the international management of hazardous substances. Her current research is supported by a National Science Foundation Graduate Research Fellow ship. She can be reached via e-mail at eckley@fas.harvard.edu.
COPYRIGHT 2005 Heldref Publications
COPYRIGHT 2005 Gale Group