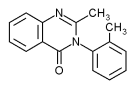
Methaqualone
Methaqualone1 is an addictive, sedative drug. more...
It is similar in effect to barbiturates, a general CNS depressant. It was used in the 1960s and 1970s as an antianxiolytic, for the treatment of insomnia, and as a sedative.
Usual effects include relaxation, euphoria, and drowsiness, also reducing heart rate and respiration. Larger doses can bring about depression, muscular miscoordination, and slurred speech.
An overdose can cause delirium, convulsions, hypertonia, hyperreflexia, vomiting, renal insufficiency, coma, and death through cardiac or respiratory arrest. It resembles barbiturate poisoning but with increased motor difficulties and a lower incidence of cardiac or respiratory depression. Toxicity is treated with diazepam and sometimes an anticonvulsant.
Methaqualone was discovered by the Indian researcher M. L. Gujiral in 1955 during an anti-malaria research program. It was marketed as a sleeping pill during the 1960s under a number of tradenames including Renoval and Melsed and in combination with an antihistamine as Mandrax. From 1965 it was sold on the US market as Quaalude, Sopor and Parest, by 1972 it was the sixth most popular sedative in the US. The name Quaalude was apparently derived from the phrase 'quiet interlude' with an added 'aa' by the manufacturers in order to elicit a more positive public recognition, as was done with the drug Maalox. It was hoped that it was a 'safer' drug than barbiturates to use for sedation; however, it was found to have similar problems of tolerance and dependence.
Quaaludes became increasingly popular as a recreational drug during the 1960s. The drug was more tightly regulated in Britain under the Misuse of Drugs Act 1971 and in the US from 1973. With its addictive nature clear, it was withdrawn from many developed markets in the 1980s, being made a Schedule I drug in the US in 1984. Up until the fall of Nicolae Ceausescu's Communist regime in the early 1990s, methaqualone (along with other sedatives) was used to pacify orphans in Romania's state-run orphanage system. Internationally, Methaqualone is a Schedule II drug under the Convention on Psychotropic Substances.
Smoking marijuana laced with methaqualone has become a major problem in South Africa, rivalling crack cocaine as the most abused hard drug. Its low price (R30.00 average against R150.00 for crack) means it is the prefered hard drug of the large low-income section of society. When smoked, usually mixed with marijuana, it causes an intensely euphoric rush.
Although methaqualone cannot be legally manufactured in the U.S. outside of research due to its Schedule I status, it is produced in other parts of the world as a legitimate pharmaceutical. It is available by prescription in Canada.
Read more at Wikipedia.org