Methionine
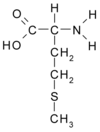
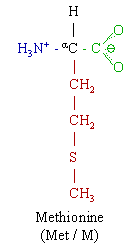
Methionine (Met, M. C5H11NO2S) is an essential nonpolar amino acid, and a lipotropic. more...
Methionine and cysteine are the only sulfur-containing proteinogenic amino acids. The methionine derivative S-adenosyl methionine (SAM) serves as a methyl donor. Methionine plays a role in cysteine, carnitine and taurine synthesis by the transsulfuration pathway, lecithin production, the synthesis of phosphatidylcholine and other phospholipids. Improper conversion of methionine can lead to atherosclerosis. Methionine is a chelating agent.
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code (tryptophan, encoded by UGG, is the other). The codon AUG is also significant, in that it carries the "Start" message for a ribosome to begin protein translation from mRNA. As a consequence, methionine is incorporated into the N-terminal position of all proteins in eukaryotes and archaea during translation, although it is usually removed by post-translational modification. Methionine can also occur at other positions in the protein.
Foods containing methionine include fruits, vegetables, and legumes. High levels of methionine can be found in spinach, green peas, corn, navel and mandarin oranges, peanuts, pistachios, macadamia nuts, kidney beans, black turtle beans, tofu, and tempeh.
Biosynthesis
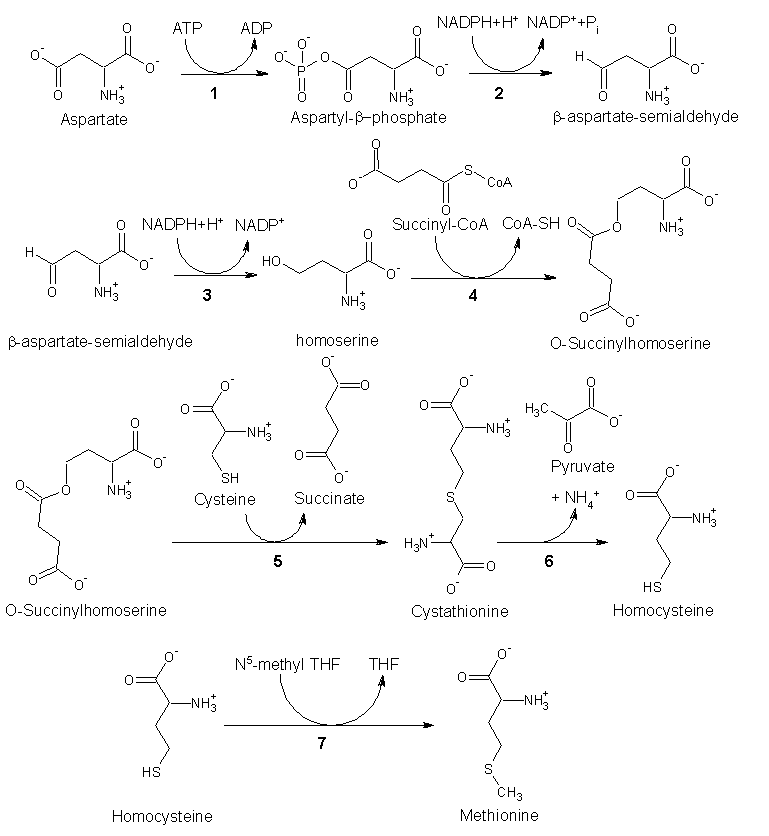
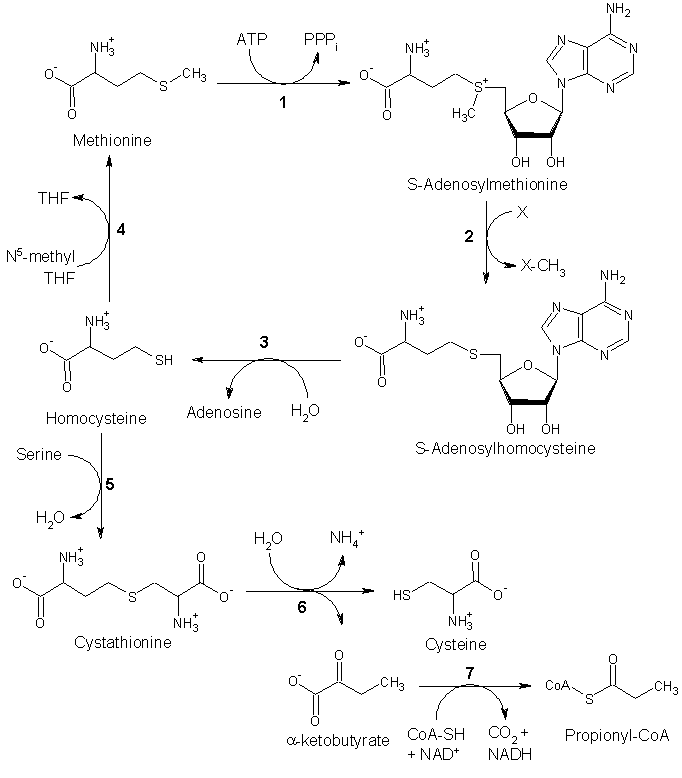
Since methionine is an essential amino acid, it cannot be synthesized in humans. However, in plants and microorganisms, methionine is synthesized from aspartic acid and cysteine. First, aspartic acid is converted to β-aspartyl-semialdehyde, an important intermediate in the biosynthesis of methionine, lysine, and, threonine. Of homoserine by homoserine acyltransferase, puts a good leaving group on homoserine allowing it to react with cysteine to produce cystathionine. Enzymatic cleavage of cystathionine yilds homocysteine, which can then be methylated by folates to give methionine. Both cystathionine-γ-synthase and cystathionine-β-lyase require Pyridoxyl-5'-phosphate as a cofactor, while homocysteine methyltransferase requires Vitamin B12 as a cofactor.
</li>
</ol>
<p></p>
<h2>Other Biochemical Pathways</h2>
<p>Although mammals cannot synthesize methionine, they can still utilize it in a variety of biochemical pathways:</p>
<p><img src=)
Methionine is converted to S-adenosylmethionine (SAM) by (1) methionine adenosyltransferase. SAM serves as a methyl-donor in many (2) methyltransferase reactions and is conveted to S-adenosylhomocysteine (SAH). (3) adenosylhomocysteinase converts SAH to homocysteine.
Read more at Wikipedia.org