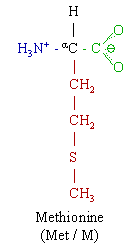
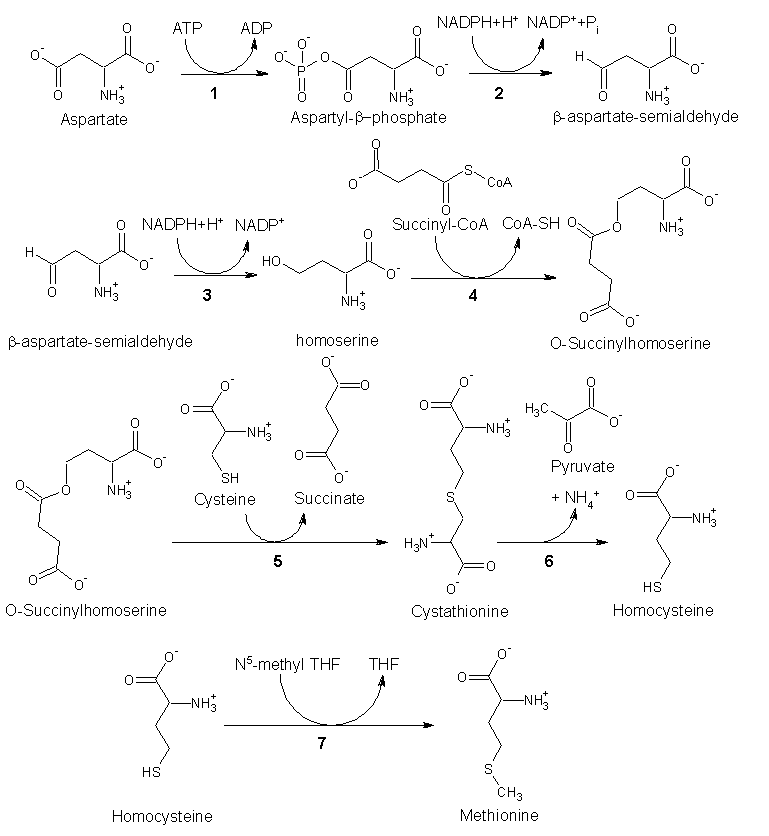
ABSTRACT Bacteriorhodopsin (BR) is an integral membrane protein, which functions as a light-driven proton pump in Halobacterium salinarum. We report evidence that one or more methionine residues undergo a structural change during the BR->M portion of the BR photocycle. Selenomethionine was incorporated into BR using a cell-free protein translation system containing an amino acid mixture with selenomethionine substituted for methionine. BR->M FTIR difference spectra recorded for unlabeled and selenomethionine-labeled cell-free expressed BR closely resemble the spectra of in vivo expressed BR. However, reproducible changes occur in two regions near 1284 and 900 cm^sup -1^ due to selenomethionine incorporation. Isotope labeled tyrosine was also co-incorporated with selenomethionine in order to confirm these assignments. Based on recent x-ray crystallographic studies, likely methionines which give rise to the FTIR difference bands are Met-118 and Met-145, which are located inside the retinal binding pocket and in a position to constrain the motion of retinal during photoisomerization. The assignment of methionine bands in the FTIR difference spectrum of BR provides a means to study methionine-chromophore interaction under physiological conditions. More generally, combining cell-free incorporations of selenomethionine into proteins with FTIR difference spectroscopy provides a useful method for investigating the role of methionines in protein structure and function.
REFERENCES
Bagley, K., G. Dollinger, L. Eisenstein, A. K. Singh, and L. Zimanyi. 1982. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc. Natl. Acad. Sci. USA. 79:4972-4976.
Barth, A., and W. Mantele. 1998. ATP-Induced phosphorylation of the sarcoplasmic reticulum Ca2+ ATPase: molecular interpretation of infrared difference spectra. Biophys. J. 75:538-544.
Bergo, V., E. N. Spudich, K. L. Scott, J. L. Spudich, and K. J. Rothschild. 2000. FTIR analysis of the S11540 intermediate of sensory rhodopsin II: Asp73 is the Schiff base proton acceptor. Biochemistry. 39:2823-2830.
Bousche, O., E. N. Spudich, J. L. Spudich, and K. J. Rothschild. 1991. Conformational changes in sensory rhodopsin I: similarities and differences with bacteriorhodopsin, halorhodopsin, and rhodopsin. Biochemistry. 30:5395-5400.
Braiman, M. S., T. Mogi, T. Marti, L. J. Stem, H. G. Khorana, and K. J. Rothschild. 1988a. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 27:8516-8520.
Braiman, M. S., T. Mogi, L. J. Stem, N. R. Hackett, B. H. Chao, H. G. Khorana, and K. J. Rothschild. 1988b. Vibrational spectroscopy of bacteriorhodopsin mutants. I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 3:219-229.
Brown, L. S., Y. Got, M. Sheves, Y. Yamazaki, A. Maeda, R. Needleman, and J. K. Lanyi. 1994. The retinal Schiff base-counterion complex of bacteriorhodopsin: changed geometry during the photocycle is a cause of proton transfer to aspartate 85. Biochemistry. 33:12001-12011.
Edman, K., P. Nollert, A. Royant, H. Belrhali, E. Pebay-Peyroula, J. Hajdu, R. Neutze, and E. M. Landau. 1999. High-resolution X-ray structure of an early intermediate in the bacteriorhodopsin photocycle. Nature. 401:822-826 [see comments].
Engelhard, M., B. Scharf, and F. Siebert. 1996. Protonation changes during the photocycle of sensory rhodopsin II from Natronobacterium pharaonis. FEBS Lett. 395:195-198.
Facciotti, M. T., S. Rouhani, F. T. Burkard, F. M. Betancourt, K. H. Downing, R. B. Rose, G. McDermott, and R. M. Glaeser. 2001. Structure of an early intermediate in the M-state phase of the bacteriorhodopsin photocycle. Biophys. J. 81:3442-3455.
Foerstendorf, H., C. Benda, W. Gartner, M. Storf, H. Scheer, and F. Siebert. 2001. FTIR studies of phytochrome photoreactions reveal the C=O bands of the chromophore: consequences for its protonation states, conformation, and protein interaction. Biochemistry. 40:14952-14959.
Greenhalgh, D. A., D. L. Farrens, S. Subramaniam, and H. G. Khorana. 1993. Hydrophobic amino acids in the retinal-binding pocket of bacteriorhodopsin. J. Biol. Chem. 268:20305-20311.
Grunenberg, A., and D. Bougeard. 1986. The observed and calculated vibrational spectra of DL-methionine in the study of the solid state phase transition. Ber. Bunsenges. Phys. Chem. 90:485-492.
Grunenberg, A., and D. Bougeard. 1987. Vibrational spectra and conformational phase transition of crystalline L-methionine. J. Mol. Struct. 160:27-36.
Kigawa, T., E. Yamaguchi-Nunokawa, K. Kodama, T. Matsuda, T. Yabuki, N. Matsuda, R. Ishitani, 0. Nureki, and S. Yokoyama. 2001. Selenomethionine incorporation into a protein by cell-free synthesis. J. Struct. Funct. Genomics. 2:27-33.
Kim, S., C. A. Sacksteder, K. A. Bixby, and B. A. Barry. 2001. A reaction-- induced FT-IR study of cyanobacterial photosystem I. Biochemistry. 40:15384-15395.
Liu, X., M. J. Lee, M. Coleman, P. Rath, A. Nilsson, W. B. Fischer, M. Bizounok, J. Herzfeld, W. F. Karstens, J. Raap, J. Lugtenburg, and K. J. Rothschild. 1998. Detection of threonine structural changes upon formation of the M- intermediate of bacteriorhodopsin: evidence for assignment to Thr-89. Biochim. Biophys. Acta. 1365:363-372.
Liu, X.-M., S. Sonar, C.-P. Lee, M. Coleman, U. L. Rajbhandary, and K. J. Rothschild. 1995. Site-directed isotope labeling and FTIR spectroscopy: Assignment of tyrosine bands in the bRiM difference spectrum of bacteriorhodopsin. Biophys. Chem. 56:63-70.
Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999a. Structural changes in bacteriorhodopsin during ion transport at 2 angstrom resolution. Science. 286:255-261.
Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999b. Structure of bacteriorhodopsin at 1.55 A resolution. J. Mol. Biol. 291:899-911.
Maeda, A., J. Sasaki, Y. J. Ohkita, M. Simpson, and J. Herzfeld. 1992a. Tryptophan perturbation in the L intermediate of bacteriorhodopsin: fourier transform infrared analysis with indole-15N shift. Biochemistry. 31:12543-12545.
Maeda, A., J. Sasaki, Y. Shichida, T. Yoshizawa, M. Chang, B. Ni, R. Needleman, and J. K. Lanyi. 1992b. Structures of aspartic acid-96 in the L and N intermediates of bacteriorhodopsin: analysis by Fourier transform infrared spectroscopy. Biochemistry. 31:4684-4690.
Messing, J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:20-78.
Mogi, T., L. J. Stem, N. R. Hackett, and H. G. Khorana. 1987. Bacteriorhodopsin mutants containing single tyrosine to phenylalanine substitutions are all active in proton translocation. Proc. Natl. Acad. Sci. USA. 84:5595-5599.
Parker, F. S. 1983. Applications of infrared, Raman and resonance Raman spectroscopy in biochemistry. Plenum Press, New York. 550.
Popot, J. L., S. E. Gerchman, and D. M. Engelman. 1987. Refolding of bacteriorhodopsin in lipid bilayers. A thermodynamically controlled twostage process. J. Mol. Biol. 198:655-676.
Promega. 1996. Protein Translation In Vitro. Protocols and Application Guide, 3rd ed. Promega Corp., Madison. 261-276.
Roepe, P., P. L. Ahl, S. K. Das Gupta, J. Herzfeld, and K. J. Rothschild. 1987. Tyrosine and carboxyl protonation changes in the bacteriorhodopsin photocycle. 1. M412 and L550 intermediates. Biochemistry. 26:6696-6707.
Roepe, P., D. Gray, J. Lugtenburg, E. M. M. van den Berg, J. Herzfeld, and K. J. Rothschild. 1988. FTIR Evidence for Tryptophan Perturbations during the Bacteriorhodopsin Photocycle. J. Am. Chem. Soc. 110: 7223-7224.
Rothschild, K. J., 0. Bousch6, M. S. Braiman, C. A. Hasselbacher, and I L. Spudich. 1988. Fourier transform infrared study of the halorhodopsin chloride pump. Biochemistry. 27:2420-2424.
Rothschild, K. J., P. Roepe, P. L. Ahl, T. N. Earnest, R. A. Bogomolni, S. K. Das Gupta, C. M. Mulliken, and J. Herzfeld. 1986. Evidence for a tyrosine protonation change during the primary phototransition of bacteriorhodopsin at low temperature. Proc. Natl. Acad. Sci. USA. 83:347-351.
Rothschild, K. J., and S. Sonar. 1995. Bacteriorhodopsin: New biophysical perspectives. In CRC handbook of organic photochemistry and photobiology. Horspool WM and P-S. Song, editors. CRC Press, Inc, London. 1521-1544.
Rothschild, K. J., M. Zagaeski, and W. A. Cantore. 1981. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem. Biophys. Res. Commun. 103: 483-489.
Shepherd, L., and R. E. Huber. 1969. Some chemical and biochemical properties of selenomethionine. Can. J. Biochem. 47:877-881. Siebert, F., and W. Maentele. 1983. Investigation of the primary
photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur. J. Biochem. 130:565-573.
Smith, S. O., M. S. Braiman, A. B. Myers, J. A. Pardoen, J. M. L. Courtin, C. Winkel, J. Lugtenburg, and R. A. Mathies. 1987. Vibrational analysis of the all-trans-retinal chromophore in light-adapted bacteriorhodopsin. J. Am. Chem. Soc. 109:3108-3125.
Sonar, S., C. P. Lee, M. Coleman, N. Patel, X. Liu, T. Marti, H. G. Khorana, U. L. Rajbhandary, and K. Rothschild. 1994. Site-directed isotope labelling and FTIR spectroscopy of bacteriorhodopsin. Nat. Struct. Biol. 1:512-517.
Tamba, M., B. Righetti, and R. Badiello. 1973. The uv and ir spectra of thio- and selenoamino acids. Atti Ist. Veneto Sci., Lett. Arti. Classe di Scienze Matematiche e Naturali. 131:105-118.
van Thor, J. J., A. J. Pierik, I. Nugteren-Roodzant, A. Xie, and K. J. Hellingwerf. 1998. Characterization of the photoconversion of green fluorescent protein with FTIR spectroscopy. Biochemistry. 37: 16915-16921.
Walter, T. J., and M. S. Braiman. 1992. FTIR difference spectroscopy of halorhodopsin in the presence of different anions. In Structure and Functions of Retinal Proteins. Rigaud JL, editor. 233-236.
Vladislav Bergo, Sergey Mamaev, Jerzy Olejnik, and Kenneth J. Rothschild
Physics Deptartment and Molecular Biophysics Laboratory, Boston University, Boston, Massachusetts 02215
Submitted June 18, 2002, and accepted for publication October 8, 2002.
Address reprint requests to Kenneth J. Rothschild, Physics Dept. and Molecular Biophysics Laboratory, 590 Commonwealth Ave., Boston, MA 02215. Tel.: 617-353-2603; Fax: 617-353-5167; E-mail: kjr@bu.edu.
Copyright Biophysical Society Feb 2003
Provided by ProQuest Information and Learning Company. All rights Reserved