* Nemaline (rod) myopathy is a congenital muscle disease with a wide spectrum of phenotypes, ranging from forms with neonatal onset and fatal outcome to asymptomatic forms. An adult-onset variant is characterized by large numbers of rod-containing myofibers, numerous rods per affected myofiber, and the absence of specific structural abnormalities typical of other muscle diseases. Few cases fulfilling these criteria have been described in the literature. Rare cases have had an associated inflammatory component, and the majority of these have occurred in patients with an underlying immunologic disorder. We present an unusual case of an immunologically competent 65-year-old man with late-onset nemaline myopathy, who was previously diagnosed with an inflammatory myopathy based on a muscle biopsy that contained chronic inflammation. His symptoms consisted of a 2-year history of progressive proximal muscle weakness; his family history was unremarkable. A neurologic examination confirmed the presence of bilateral proximal muscle weakness, normal sensation, and decreased upper and lower extremity reflexes. Creatine kinase levels were normal, and electromyographic findings indicated a myopathic process. A modified trichrome stain of the right biceps muscle revealed granular, basophilic, centrally located rods in the atrophic myofibers. Ultrastructurally, these myofibers contained osmiophilic rectangular structures with a latticelike appearance typical of nemaline myopathy. This case illustrates that adult-onset nemaline myopathy, although rare, should be considered in the differential diagnosis of an inflammatory myopathy. (Arch Pathol Lab Med. 1997;121:1210-1213)
Nemaline (rod) myopathy is an uncommon muscle disease with a wide spectrum of phenotypes, including a congenital form with neonatal onset and fatal outcome, a mild congenital form with slowly progressive or nonprogressive weakness, and a sporadic adult-onset form.1 The adult-onset variant is characterized by large numbers of rod-containing myofibers, numerous rods per affected myofiber, and the absence of specific structural abnormalities typical of other muscle diseases or of associated malformations typical of the congenital forms.2 Few cases fulfilling these criteria have been reported in the literature. Approximately half of the patients with this variant have associated immunologic disorders,3-11 prompting some authors to postulate a role for the immune system in the pathogenesis of this disorder.3,4,7,9 When chronic inflammatory infiltrates are present in hematoxylin-eosinstained specimens from patients thought clinically to have an inflammatory myopathy, recognizing and differentiating this entity from a primary inflammatory myopathy can be difficult.
We present an unusual case of adult-onset nemaline myopathy in an immunocompetent patient previously diagnosed with an inflammatory myopathy and review the literature on adult-onset nemaline myopathy.
REPORT OF A CASE
A 65-year-old man presented with progressive proximal weakness of approximately 2 years duration. An initial muscle biopsy contained a focus of chronic inflammation, and the patient received a 1-week course of steroids without benefit. At the time of this evaluation, 1 year later, he was unable to rise from a chair or to lift his arms over his head. He had experienced several recent falls secondary to lower extremity weakness, and he reported occasional difficulty swallowing solid foods. His medical history was significant for hypercholesterolemia, coronary artery disease requiring bypass grafting, and a cerebrovascular accident from which he functionally recovered completely. There was no family history of neuromuscular disease.
A neurologic examination confirmed the presence of generalized weakness, which was most prominent in proximal muscle groups (grade 2/5). The patients reflexes were decreased, and his sensory and cranial nerve examinations were unremarkable.
Numerous laboratory parameters, including aldolase, creatine kinase, C-reactive protein, erythrocyte sedimentation rate, rheumatoid factor, antinuclear and anti-SS-A (Ro) antibodies, and thyrotropin, were normal. A cervical spine magnetic resonance imaging scan was unremarkable. Electromyography revealed fibrillation potentials, as well as highly polyphasic motor unit potentials of increased duration and decreased amplitude in muscles from the upper and lower extremities. Nerve conduction studies were normal. A biopsy of the right biceps muscle was performed.
PATHOLOGIC FINDINGS
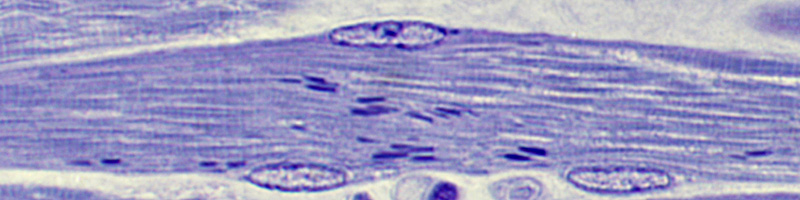
Hematoxylin-eosin-stained sections of the formalinfixed, paraffin-embedded material from the right biceps muscle biopsy revealed prominent atrophy, marked variation in muscle fiber size, nuclear bag formation, increased numbers of central nuclei, increased endomysial fibrous tissue, and rare foci of chronic inflammation (Fig 1). Enzyme histochemistry and special stains, including acid and alkaline phosphatase, esterase, modified Gomori's trichrome, nicotinamide-adenine dinucleotide (reduced form), cytochrome oxidase, periodic acid-Schiff, oil red O, sulfonated Alcan blue, and adenosine triphosphatase at pH 4.6 and 9.8, were performed on unfixed, snap-frozen material. The modified trichrome stain showed innumerable, granular, red rods in approximately 60% of the muscle fibers, most of which were atrophic. These were predominantly centrally located (Fig 2). Scattered angular, atrophic, esterase-positive muscle fibers were also present. Most of the atrophic myofibers were type 2 myofibers; however, rods were present in both type 1 and type 2 muscle fibers.
Electron microscopic examination was performed on glutaraldehyde-fixed, uranyl acetate- and lead citratestained material from the biopsy of the right biceps muscle. Osmiophilic, rectangular structures with a latticelike appearance typical of nemaline rod bodies emanated from the Z-discs of affected muscle fibers (Fig 3). No other abnormalities were present.
COMMENT
Few well-documented cases of adult-onset nemaline myopathy have been described in the literature (Table). The presence of nemaline rods alone in an adult is insufficient for a diagnosis of adult-onset nemaline myopathy. These structures rarely may be seen in a number of other neuromuscular disorders, including mitochondrial myopathies,12,13 polymyositis,14 spinal progressive muscular atrophy,15 and acute alcoholic myopathy.16 They can also occur as a component of a mixed myopathy17-21 and are thought to represent a nonspecific reaction to injury in these cases.14 Nemaline rods have also been described in patients with no skeletal muscle symptomatology.22-24 An accurate diagnosis requires the presence of large numbers of nemaline rods in a symptomatic patient, as well as the absence of findings typically seen in other myopathies.2 Cases reported as examples of adult-onset nemaline myopathy, including those in which the patient's first symptoms were present in childhood,25,26 in adult patients with other skeletal abnormalities suggestive of congenital nemaline myopathy27,28 and in cases where fewer than 10% of muscle fibers contained nemaline rods29 have caused some authors to question the existence of late-onset nemaline myopathy as a distinct entity.30,31 However, the homogeneous clinical and pathologic features present in well-documented cases, as well as the inability to diagnose another neuromuscular disorder in these patients, support the consideration of adult-onset nemaline myopathy as a distinct entity.31
The salient clinical and pathologic features of well-defined cases of adult-onset nemaline myopathy are summarized in the Table. Patients described in case reports have ranged in age from 23 to 79 years (mean, 45 years), and there is a slight male predominance. Most patients present with proximal muscle weakness, which is typically slowly progressive and often unresponsive to steroid therapy. Respiratory failure is the most common cause of death in these patients.6,10,32 Creatine kinase levels are normal to mildly elevated and electromyography discloses a myopathic pattern of injury. Other laboratory findings that have been variably reported include an increased erythrocyte sedimentation rate,2,9,33 positive antinuclear antibodies,10 and increased serum immunoglobulins.6,7,10
Pathologic findings include muscle fiber atrophy, as well as intracytoplasmic and, rarely, intranuclear 26 nemaline rods. These rods, which are derived from Z-band material and are composed of actin34 and alpha-actinin,2 may be located either subsarcolemmally or centrally. In most cases, rods are seen in both type 1 and type 2 muscle fibers; however, occasionally preferential involvement of type 2 fibers is seen.4,5,9,35 A reversal of the type 1 to type 2 ratio has also been described.2,32 Since nemaline rods have been described in muscles from a variety of anatomic sites, including both type 1 and type 2 predominant muscles, these findings are of uncertain significance.
Our case is similar in certain respects to previously reported cases of adult-onset nemaline myopathy; our patient presented with progressive, predominantly proximal muscle weakness, which did not improve with steroid therapy; a normal creatine kinase level; and a myopathic pattern on electromyography. The presence of chronic inflammatory cells in the patient's first biopsy, in which only a hematoxylin-eosin stain was performed, suggested the possibility of an inflammatory myopathy. However, a subsequent extensive clinical and optimal pathologic examination of a second biopsy failed to reveal findings typical of primary inflammatory myopathies, such as myofiber necrosis and degeneration, vasculitis, and rimmed vacuoles and tubulofilamentous inclusions typical of inclusion body myositis. Inflammation, present as perivascular or small foci of endomysial chronic inflammation, has been described in six other patients with adult-onset nemaline myopathy.8,9,11,30,33 These cases illustrate the importance of performing a trichrome stain on frozen tissue or electron microscopy on biopsies from patients suspected of having an inflammatory myopathy; the characteristic nemaline rods are seen poorly, if at all, in hematoxylin-eosinstained sections.
Interestingly, approximately one half of patients with adult-onset nemaline myopathy, including four of the six patients with chronic inflammation on biopsy, have had an identified underlying immunologic disorder. Associated conditions include human immunodeficiency virus infection,3-5,8,9,11 human T-cell lymphotropic virus-2 infection,9 monoclonal gammopathy,6,7 and primary hypothyroidism in a patient with antinuclear antibodies.10 These findings, as well as the presence of immunoglobulin deposits in the sarcolemma of one patient's muscle7 and the response of several of these patients to immunosuppressive therapy,5,7,8,11 have prompted some authors to suggest a role for paraproteins, a defect in cellular immunity, or autoimmunity in the pathogenesis of adult-onset nemaline myopathy.3,4,7-9 Gonzales et al8 postulated that adult-onset nemaline myopathy may represent part of a spectrum of inflammatory myopathic processes with polymyositis and autoimmune myopathies at one end (in patients with minimal defects in cellular immunity) and nemaline myopathy without inflammation (in patients with severe defects in cellular immunity) at the other. This hypothesis is supported by the coexistence of nemaline rods and polymyositis14 and by the finding of both disorders in patients with human immunodeficiency virus infection.11 Despite an extensive workup, no evidence of altered immunity was present in our patient, suggesting that defects in immunity may represent only one of several possible pathogenetic mechanisms responsible for this disorder.
In summary, adult-onset nemaline myopathy, although rare, should be considered in the differential diagnosis in adult patients with proximal muscle weakness, especially in those with underlying immunologic disorders. The characteristic nemaline rods are easily missed in routine hematoxylin-eosin-stained sections; therefore, a modified Gomori's trichrome stain on cryostat sections with possible confirmatory electron microscopy should be performed in the evaluation of these cases. Finally, the presence of inflammatory infiltrates in a subset of these patients and the association of adult-onset nemaline myopathy with immune-mediated disorders suggest that this entity may represent a form of inflammatory myopathy.
References
1. Kuitunen P, Rapola J, Noponen AL, Donner M. Nemaline myopathy: report of four cases and review of the literature. Acta Paediatr Scand. 1972;61353-361.
2. Paulus W, Peiffer J, Becker I, Roggendorf W, Schumm F Adult-onset rod disease with abundant intranuclear rods. J Neurol.1988;235:343-347. 3. Cabello A, Martinez-Martin P, Gutierrez-Rivas E, Madero S. Myopathy with nemaline structures associated with HIV infection.JNeurol. 1990;237:64-66. Letter.
4. Dalakas MC. Progressive nemaline (rod) myopathy associated with HIV infection. N Engl/ Med. 1987;317:1602-1603. Letter. 5. Dwyer BA, Mayer RF, Lee SC. Progressive nemaline (rod) myopathy as a presentation of human immunodeficiency virus infection. Arch Neurol.1992;49: 440. Letter.
6. Engel WK, Oberc MA. Abundant nuclear rods in adult-onset rod disease.J Neuropathol Exp Neurol.1975;34:119-131.
7. Eymard B, Brouet JC, Chevallay M, Bussel A, Fardeau M. Late-onset rod
myopathy associated with monoclonal gammopathy. Neuromuscul Disord.1993; 3:557-560.
8. Gonzales MF, Olney RK, So YT, et al, Subacute structural myopathy associated with human immunodeficiency virus infection. Arch Neurol. 1988;45: 585-587.
9. Maytal J, Horowitz S, Lipper S, Poiesz B, Wang CY, Siegal FP. Progressive nemaline rod myopathy in a woman coinfected with HIV-1 and HTLV-2. Mt Sinai J Med. 1993;60:242-246.
10. Reyes MG, Tal A, Abrahamson D, Schwartz M. Nemaline myopathy in an adult with primary hypothyroidism. Can/Neurol Sci. 1986;13:117-119. 11. Simpson DM, Bender AN. Human immunodeficiency virus-associated myopathy: analysis of 11 patients. Ann Neurol. 1988;24:79-84.
12. Danon MJ, Giometti CS, Manaligod JR, Perurena OH, Skosey IL. Adultonset nemaline rods in a patient treated for suspected dermatomyositis: study with two-dimensional electrophoresis. Arch Neurol.1981;38:761-766.
13. Hanna W, Henderson RD. Nemaline rods in cricopharyngeal dysphagia. Am J Clin Pathol. 1980;74:186-191.
14. Cape CA, Johnson WW, Pitner SE. Nemaline structures in polymyositis: a nonspecific pathological reaction of skeletal muscles. Neurology. 1970;20:494502.
15. Konno H, Iwasaki Y, Yamamoto T, Inosaka T. Nemaline bodies in spinal progressive muscular atrophy. Acta Neuropathol. 1987;74:84-88.
16. Martinez AJ, Hooshmand H, Faris AA. Acute alcoholic myopathy: enzyme histochemistry and electron microscope findings. J Neurol Sci.1973;20:245-252. 17. Bethlem J, Arts WF, Dingemans KP. Common origin of rods, cores, miniature cores, and focal loss of cross-striations. Arch Neurol.1978;35:555-566.
18. Heffernan LP, Rewcastle NB, Humphrey JG. The spectrum of rod myopathies. Arch Neurol.1968;18:529-542.
19. Lindsey JR, Hopkins If, Clark DB. Pathology of nemaline myopathy: studies of two adult cases including an autopsy. ) Neuropathol Exp Neurol. 1967;26: 129-130.
20. Pourmand R, Azzarelli B. Adult-onset of nemaline myopathy, associated with cores and abnormal mitochondria. Muscle Nerve. 1994;17:1218-1220. 21. Seitz RJ, Toyka KV, Wechsler W. Adult-onset mixed myopathy with nemaline rods, minicores, and central cores: a muscle disorder mimicking polymyositis. J Neurol.1984;231:103-108.
22. Meier C, Gertsch M, Zimmerman A, Voellmy W, Geissbhler J. Nemaline myopathy presenting as cardiomyopathy. N Engl J Med. 1983;308:1536-1537. Letter.
23. Meier C, Voellmy W, Gertsch M, Zimmerman A, Geissbihler J. Nemaline myopathy appearing in adults as cardiomyopathy: a clinicopathologic study. Arch Neurol.1984;41:443-445.
24. Meltzer HY, McBride E, Poppei RW. Rod (nemaline) bodies in the skeletal muscle of an acute schizophrenic patient. Neurology.1973;23:769-780. 25. Rohkamm R. Late onset nemaline (rod) myopathy. Clin Neuropathol. 1986; 5:120121. Abstract.
26. Stoessl AJ, Hahn AF, Mallot D, Jones DT, Silver MD. Nemaline myopathy with associated cardiomyopathy: report of clinical and detailed autopsy findings. Arch Neurol.1985;42:1084-1086.
27. Dodson RF, Crisp GO, Nicotra B, Munoz L, Albright CD. Rod myopathy with extensive systemic and respiratory muscular involvement. Ultrastruct Pathol. 1983;5:129-133.
28. Hopkins IJ, Lindsey JR, Ford FR. Nemaline myopathy: a long-term clinicopathologic study of affected mother and daughter. Brain. 1966;89:299-313. 29. Greenwood SM, Viozzi Fl. Nemaline myopathy. Arch Pathol Lab Med. 1978;102:196-200.
30. Engel AG. Late-onset rod myopathy (a new syndrome?): light and electron microscopic observations in two cases. Mayo Clin Proc. 1966;713-741. 31. Palmucci L, Doriguzzi C, Mongini T, Chiado-Piat L. Adult onset nemaline myopathy: a distinct nosologic entity? Clin Neuropathol.1993;12:153-155.
32. Brownell AKW, Gilbert Jj, Shaw DT, Garcia B, Wenkebach GF, Lam AKS. Adult onset nemaline myopathy. Neurology. 1978;28:1306-1309. 33. Kamieniecka Z. Late onset myopathy with rod-like particles. Acta Neurol Scand. 1973;49:547-551.
34. Yamaguchi M, Robson RM, Stromer MH. Actin filaments form the backbone of nemaline myopathy rods. Nature.1978;271:265-267. 35. Kula RW, Sher JH, Shafiq SA, Lapovsky AJ. Adult-onset rod disease: late occurrence with dementia. J Neuropathol Exp Neurol. 1978;37:646. Abstract.
36. Engel WK, Resnick )S. Late-onset rod myopathy: a newly recognized, acqui red, and progressive disease. Neurology. 1966;16:308-309. 37. Nonaka I, Ishiura S, Arahata K, Ishibashi-Ueda H, Maruyama T, li K. Progression in nemaline myopathy. Acta Neuropathol. 1989;78:484-491.
Accepted for publication April 28, 1997. From the Department of Anatomic Pathology, Cleveland (Ohio) Clinic Foundation.
Presented in part at the annual meeting of the American Association of Neuropathologists, Vancouver, British Columbia, Canada, June 1996.
Reprint requests to Department of Anatomic Pathology (L25), Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195 (Dr Prayson).
Copyright College of American Pathologists Nov 1997
Provided by ProQuest Information and Learning Company. All rights Reserved