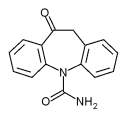
Oxcarbazepine
Oxcarbazepine (Marketed as Trileptal® by Novartis) is an anticonvulsant and mood stabilizing drug, used primarily in the treatment of epilepsy and bipolar disorder. more...
Oxcarbazepine is structurally a derivative of carbamazepine, adding an extra oxygen atom to the benzylcarboxamide group. This difference helps reduce the impact on the liver of metabolizing the drug, and also prevents the serious forms of anemia occasionally associated with carbamazepine. Aside from this reduction in side effects, it is thought to have the same mechanism as carbamazepine - sodium channel inhibition - and is generally used to treat the same conditions.
Side effects
Oxcarbazepine occasionally causes fatigue, nausea, vomiting, headache, dizziness, drowsiness, and blurred or double vision. It can cause hyponatremia, so blood sodium levels should be tested if the patient complains of severe fatigue.
History
First synthesized in 1966, it was approved for use as an anticonvulsant in Denmark in 1990. It was approved in all EU countries in 1999 and in the US in 2000.
Other Information
Oxcarbazapine may make cause oral hormonal contraceptives to be less effective.
Read more at Wikipedia.org