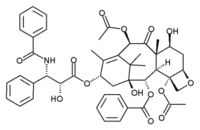
Paclitaxel
Paclitaxel (Taxol®) is a drug used in the treatment of cancer. It was discovered at Research Triangle Institute (RTI) in 1967 when Dr. Monroe E. Wall and Dr. Mansukh C. Wani isolated the compound from the bark of the Pacific yew tree, Taxus brevifolia, and noted its antitumor activity in a broad range of rodent tumors. By 1970, the two scientists had determined the structure of paclitaxel, which is extremely complex. more...
Paclitaxel has since become an effective tool of doctors who treat patients with lung, ovarian, breast cancer, and advanced forms of Kaposi's sarcoma (Saville et al 1995). It is sold under the tradename Taxol®. Together with docetaxel, it forms the drug category of the taxanes. Paclitaxel is also used for the prevention of restenosis (recurrent narrowing) of coronary stents; locally delivered to the wall of the coronary artery, a paclitaxel coating limits the growth of neointima (scar tissue) within stents (Heldman et al 2001).
History
The history of paclitaxel begins with a 1958 National Cancer Institute study that commissioned Department of Agriculture botanists to collect samples of over 30,000 plants to test for anticancer properties. Arthur S. Barclay, one of those botanists, collected 15 lbs of twigs, needles, and bark from Pacific yew trees in a forest near Mount St. Helens. Months later, in 1963, Monroe E. Wall discovered that bark extract from the Pacific yew possessed antitumor qualities, beginning to reveal the tree's hidden treasure. Soon after, Wall and his colleague Mansukh C. Wani were busy isolating and purifying plant compounds for anticancer tests in Research Triangle Park, North Carolina. In 1967 the team had isolated the active ingredient, announcing their findings at an American Chemical Society meeting in Miami Beach. Wall and Wani published their results, including the chemical structure, in a 1971 issue of the Journal of the American Chemical Society. The paper was noticed immediately by Robert A. Holton who was starting postdoctoral research at Stanford University in natural products synthesis. But, it would be several years before he dedicated his attention to synthesizing pacilitaxel at Florida State University, quelling an emerging environmental controversy; a 40-foot Pacific yew tree, which may have taken 200 years to reach that height, yields only a half gram of paclitaxel, but Holton's group perfected a four-step procedure to convert 10-deacetylbaccatin (a related compound in Pacific yew needles) into paclitaxel. In the late 1970s, Susan B. Horwitz, a molecular pharmacologist at Albert Einstein College of Medicine in New York City, unraveled the key mystery of how paclitaxel works. Largely in part of an enormous research and development effort, starting in government facilities and later in commercial labs, paclitaxel quickly became an all-time best-selling pharmaceutical. Paclitaxel was brought to the market by Bristol-Myers Squibb in 1993 as Taxol®. Annual sales peaked in 2000, reaching US$1.6 billion.
Production
Unfortunately, the Pacific yew is one of the slowest growing trees in the world. Further, the treatment of just one patient requires the cutting down and processing of six 100-year old trees. This supply problem combined with the threat to the endangered spotted owl (Strix occidentalis) has prompted researchers to develop actinobacteria from which paclitaxel-like compounds can be obtained by fermentation. Cultures of the fungus Nodulisporium sylviforme can be used to produce paclitaxel itself.
Read more at Wikipedia.org