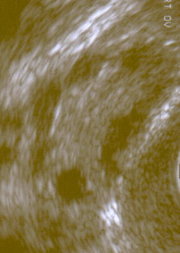
Polycystic ovarian syndrome is the most common form of anovulatory infertility.[1] Its association with menstrual disturbance and altered hormonal parameters leads many affected women of reproductive age to attend a gynaecology or infertility clinic. The aetiology of the condition is unknown, but recent evidence suggests that the principal underlying disorder is one of insulin resistance, with the resultant hyperinsulinaemia stimulating excess ovarian androgen production. Associated with the prevalent insulin resistance, these women exhibit a characteristic dyslipidaemia and a predisposition to non-insulin dependent diabetes and cardiovascular disease in later life. Thus, polycystic ovarian syndrome seems to have many of the hallmarks of the metabolic syndrome.[2-4] This article focuses on the recent change in attitudes to polycystic ovarian syndrome arising from the link with insulin resistance--a concept that not only has major implications for the health of affected women but also offers a potential for new treatments.
Methods
This article is derived from a review of recent publications in the relevant subjects of endocrinology, reproductive medicine, and gynaecology. In addition, we conducted a Medline search of "polycystic ovarian syndrome" and "metabolic syndrome in women." Our clinical experience and that of colleagues in relevant specialties further supplemented this review.
Diagnostic parameters
Diagnostic clinical features of polycystic ovarian syndrome include menstrual disturbance secondary to chronic anovulation or oligoovulation, and hirsutism or acne due to hyperandrogenaemia. Despite this classic concept, it is a heterogeneous disorder and exact diagnostic criteria remain contentious (see box). Hence, along with racial variations, the prevalence of the condition can only be estimated at between 5% and 10% of women of reproductive age.[5]
Elevated free testosterone activity, defined by the free androgen index, represents the most sensitive biochemical marker supporting the diagnosis. A raised luteinising hormone concentration, although a useful marker of the syndrome, is now less favoured as a diagnostic tool.[5] Most, but not all, subjects show a characteristic ultrasound appearance of enlarged ovaries and an increased echo dense stroma surrounded by multiple, small, peripherally situated follicles.[5] Exclusion of other possible aetiologies that may present in a similar fashion--such as late onset congenital adrenal hyperplasia, thyroid disease, hyperprolactinaemia, and androgen secreting tumours--is essential.
Aetiology
The aetiology of polycystic ovarian syndrome is uncertain. There is some evidence of autosomal transmission related to strong familial clustering. Potentially, a gene or series of genes renders the ovaries susceptible to insulin stimulation of androgen secretion while blocking follicular maturation? This genetic predisposition may be expressed as premature balding in men.[7]
The onset may occur in late childhood since many of the metabolic and endocrine features of the disorder mimic puberty.[8] Insulin resistance increases dramatically at the onset of puberty and then declines in early adulthood. Associated with this are increases in the pulse amplitude of luteinising hormone, increasing androgen concentrations, and irregular menses. Multiple, small ovarian cysts are seen on ultrasound examination and are a common and normal feature of puberty. It is therefore possible that women genetically predisposed to polycystic ovarian syndrome fail to resume normal insulin sensitivity and continue to express metabolic and endocrine features usually confined to puberty.[8]
Pathophysiology
Good evidence supports the hypothesis that decreased peripheral insulin sensitivity and consequent hyperinsulinaemia are pivotal in the pathogenesis of polycystic ovarian syndrome.[5] Peripheral insulin resistance is most evident in overweight patients: obesity and polycystic ovarian syndrome each seem to have a separate and synergistic relation with insulin resistance.[5] The exact mechanism(s) for insulin resistance is uncertain, but a post-receptor defect in adipose tissue has been identitied.[5] Despite insulin resistance in adipose and skeletal muscle, the ovary remains relatively sensitive to insulin, and both insulin and insulin-like growth factor 1 have stimulatory effects on thecal androgen production? In fact, some lean women with polycystic ovarian syndrome, who may not have insulin resistance and therefore hyperinsulinaemia, may show enhanced ovarian sensitivity to insulin. Figure 1 shows how the relative excess of insulin or enhanced ovarian sensitivity to insulin, in combination with an elevated luteinising hormone concentration, brings about thecal hyperplasia, increased androgen secretion, arrest of follicular development, and therefore anovulation along with menstrual disturbance.
[Figure 1 ILLUSTRATION OMITTED]
Insulin also acts on the liver to inhibit the production of sex hormone binding globulin and insulin-like growth factor 1 binding protein. A reduction in sex hormone binding globulin leads to an increase in the biologically available free testosterone. Thus, insulin resistance not only increases secretion of ovarian androgens but also promotes an increase in the proportion of free (active) hormone. Similarly, inhibition of production of insulin-like growth factor 1 binding protein results in an increased concentration of circulating free insulin-like growth factor 1, further enhancing ovarian androgen production.[10]
Current consensus suggests that the ovary is the principal site of excess androgen production, but some women with polycystic ovarian syndrome may have an adrenal contribution to the increased androgen production. The mechanisms for this remain obscure and are almost certainly multifactorial.[11]
Metabolism and health implications
Figure 2 shows the principal features of the polycystic ovarian syndrome. It is well recognised that visceral distribution of body fat, common in the syndrome, is of greater consequence to the metabolic effects of insulin resistance than obesity per se).[12 13] Central obesity and insulin resistance lead to an altered lipolytic response to insulin, with impaired suppression of release of free fatty acids from adipose tissue. An increased flux of free fatty acids from central sites enters the portal circulation, increasing the availability of substrate to the liver for triglyceride production. Furthermore, women with the syndrome exhibit increased activity of hepatic lipase, an enzyme responsible for the conversion of large lipoprotein particles to smaller, more atherogenic species. This explains the findings of reduced concentrations of high density lipoprotein cholesterol and increased levels of atherogenic, small, low density lipoprotein.[14]
[Figure 2 ILLUSTRATION OMITTED]
The combination of raised triglyceride and decreased high density lipoprotein is strongly linked with cardiovascular disease.[15] Discrepancies in these lipid parameters between patients with polycystic ovarian syndrome and controls matched for age and weight are evident at an early age.[2] Hence, an increased risk of cardiovascular disease due to lipid perturbances will present in early adult life. Women with polycystic ovarian syndrome also show elevated concentrations of plasminogen activator inhibitor[1,16 17] a potent inhibitor of fibrinolysis, which have been shown to predict the occurrence of myocardial infarction. Suppression of hyperandrogenaemia by use of gonadotrophin releasing hormone analogues has little effect on the insulin resistance or the dyslipidaemia, suggesting that the abnormal lipid profile is independent of the raised androgen concentrations.[5]
Important retrospective studies provide evidence of increased risk of cardiovascular disorders. A study of women thought to have polycystic ovarian syndrome who were treated with ovarian wedge resection 20-30 years earlier showed that they were four times more likely to be receiving treatment for hypertension than age and weight matched controls and seven times more likely to have a diagnosis of diabetes.[18] Studies of women undergoing coronary angiography for evaluation of chest pain found a disproportionately large number with polycystic ovaries on ultrasound scan.[19] Furthermore, on multiple linear regression analysis the presence of polycystic ovaries was independently associated with the severity of the coronary vascular disease. Models using triglyceride concentrations, waist to hip ratio, non-insulin dependent diabetes, and elevated blood pressure in women with polycystic ovarian syndrome indicate a 7.4-fold increased risk of myocardial infarction compared with age matched referents.[3] Clearly, comprehensive longitudinal studies are required if the long term implications of the syndrome for cardiovascular health are to be fully appreciated.
Current and future treatments
Women with polycystic ovarian syndrome are currently treated according to their presenting features-irregular menses, hirsutism, or infertility (table).
Irregular menses--The combined oral contraceptive pill is commonly used to regulate menses. By increasing levels of sex hormone binding globulin while decreasing androgen secretion, it reduces the circulating free testosterone activity. However, the combined pill exacerbates insulin resistance, and, since many patients are overweight and obesity is a relative contraindication, this treatment may be unsuitable.[20]
Hirsutism may be addressed by the use of the antiandrogens cyproterone acetate or spironolactone (the former used in combination with ethinyloestradiol).[21] Their principal mode of action is the inhibition of the binding of dihydrotestosterone to its receptor at the hair follicle. Beneficial effects can be seen after three months, but excessive hair growth returns soon after cessation of treatment. Cyproterone acetate may exacerbate irregularity of the menstrual cycle, and both drugs are unsuitable for use in those trying to conceive.
Infertility--For patients wishing to become pregnant, domiphene citrate may be successful in stimulating ovulation but carries an increased risk of multiple pregnancy.[22] By inhibiting the oestrogen mediated negative feedback loop at the hypothalamus, it enhances secretion of follicle stimulating hormone. Guidelines suggest that the duration of clomiphene treatment should not exceed six months because of the potential increased risk of ovarian cancer.[23] Those failing to conceive after clomiphene treatment usually respond to exogenous gonadotrophins, but this requires intensive monitoring to reduce the risk of multiple conceptions.
Alternatives to medical treatment include laser or electrocautery of the ovary. This is often used as a last resort, is not available in all centres, and is difficult with obese patients. Although effective in aiding ovulation and regulating menses, its beneficial effects are usually short term.
Insulin resistance
As the principal underlying defect in polycystic ovarian syndrome seems to be insulin resistance, the most appropriate treatment for all clinical presentations may be one that specifically addresses this problem.
Weight reduction has multiple benefits for obese women with polycystic ovarian syndrome.[24] The resultant reduction in insulin resistance corrects the hormonal imbalance, promotes ovulation and regular menses, and improves the metabolic consequences of the disorder. Weight loss should therefore be encouraged, but it seems to be hard to achieve for this group of patients.
Insulin sensitising agents--Recent trials have investigated the effect of such agents on polycystic ovarian syndrome.[16 17 24-33] Metformin, a biguanide often used in non-insulin dependent diabetes, has been the most commonly used. Troglitazone, a thiazolidinedione that improves muscle insulin sensitivity, has also been studied[17 27] but has recently been removed from the market because of adverse effects on hepatic function. Trials to date have included only small numbers of subjects, but results have been promising, with most showing reductions in concentrations of fasting serum insulin, androgen, and luteinising hormone)[16 17 26 28 29 31-33] In addition, circulating concentrations of sex hormone binding globulin increased, resulting in less bioactively available testosterone. Preliminary evidence indicates that treatment of obese women with polycystic ovarian syndrome with metformin restores regular menstrual cycles and ovulation[26 30-32] Whether insulin sensitising agents can modify the vascular risk factors associated with the syndrome remains to be seen, but reductions in Lp (a) lipoprotein and plasminogen activator inhibitor 1 have been observed.[16 17] Additionally, some studies have reported that treated subjects have shown some weight loss despite continuation of their normal diet and lifestyle,[16 26] and others have demonstrated a reduction in central obesity[16 29 33]
Thus, treatments targeting the key factor in the disorder may not only resolve the gynaecological problems with which the syndrome presents, but also reduce the risk of vascular disease in later life. There is now an urgent need for randomised, placebo controlled trials to assess the potential benefits of these treatments for women's health.
Funding: This review was written under the tenure of a Scotfish Office Home and Health Department (SOHHD) grant.
Conflict of interest: None.
[1] Frank S. Polycystic ovary syndrome. N Engl J Med 1995;333:853-61.
[2] Talbott E, Guzick D, Clerici A, Berga S, Detre K, Weimer K, et al. Coronary heart disease risk factors in women with polycystic ovarian syndrome. Arterioscler Thromb Vasc Biol 1995;15:821-6.
[3] Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction: evaluated from a risk factor model based on a prospective study of women. Acta Obstet Gynecol Scand 1992;71:599-604.
[4] Conway G, Agrawal R, Betteridge DJ, Jacobs HS. Risk factors for coronary heart disease in lean and obese women with PCOS. Clin Endocrinol 1992;37:119-25.
[5] Dunaif A. Insulin resistance and the polycystic ovarian syndrome: mechanisms and implications for pathogenesis. EndocrRev 1997;18:774-800.
[6] Nestler JE. Insulin regulation of human ovarian androgens. Hum Reprod 1997;12(suppl):52-62.
[7] Carey AH, Chan KL, Short F, White D, Williamson R, Franks S. Evidence for a single gene effect causing polycystic ovaries and male pattern baldness. Clin Endocrinol 1993;38:653-8.
[8] Nobels F, Dewailley D. Puberty and polycystic ovary syndrome: the insulin/insulin-like growth factor hypothesis. Fertil Steril 1992;58:655-63.
[9] Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjo T Regulation of androgen production in cultured human thecal cells by IGF-1 and insulin. Fertil Steril 1993;59:323-31.
[10] Cataldo NA. Insulin like growth factor binding proteins: do they play a role in polycystic ovary syndrome? Endocrinology 1997;15:123-36.
[11] Gonzalez E Adrenal involvement in polycystic ovary syndrome. Semin Reprod Endocrinol 1997;15:137-57.
[12] Douchi T, Ijuin H, Nakamura S, Oki T, Yamamoto S, Nagata Y. Body fat distribution in women with polycystic ovary syndrome. Obstet Gynecol 1995;86:516-9.
[13] Despres JP, Moorjani S, Lupein P, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins and cardiovascular disease. Arteriosclerosis 1990;10:497-511.
[14] Pirwani I, Sattar N, Packard CJ, Wallace AM, Fleming R, Greer IA. Lipoprotein subfraction changes in women with oligomenorrhea: relationship to metabolic, hormonal and anthropometric indices. J Soc Gynecol Invest 1997;4(suppl):90A.
[15] Wild R. Metabolic aspects of polycystic ovary syndrome. Semin Reprod Endocrinol 1997;15:105-10.
[16] Velazquez EM, Mendoza SG, Wang P, Glueck CJ. Metformin therapy is associated with decrease plasma plasminogen activator inhibitor-1, lipoprotein (a), and immunoreactive insulin levels in patients with polycystic ovary syndrome. Metabolism 1997;46:454-7.
[17] Ehrmann D, Schneider DJ, Sobel BE, Cavaghan MK, Imperial J, Sturis J, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1997;82:2108-16.
[18] Dahlgreen E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril 1992;57:505-13.
[19] Birdsall M, Farquar C, White H. Association between polycystic ovaries and the extent of coronary artery disease in women having cardiac catheterisation. Ann Intern Med 1997;126:32-5.
[20] Sheu WH, Hsu CH, Chen YS, Jeng CY, Fuh MM. Prospective evaluation of insulin resistance and lipid metabolism in women receiving the oral contraceptives. Clin Endocrinol 1994;40:249-55.
[21] Erenus M, Yucelton D, Gurbuz O, Durmusoglu F, Pekin S. Comparison of spironolactone-oral contraceptive versus cyproterone acetate-estrogen regimens in the treatment of hirsutism. Fertil Steril 1996;66:216-9.
[22] Kettel LM, Hummel WP. Ovulation induction in the oestrogenised anovulatory patient. Semin Reprod Endocrinol 1996;14:309-15.
[23] Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumours in a cohort of infertile women. N Engl J Med 1994;331:771-6.
[24] Crave JC, Fimbel S, Lejeune H, Cuguardey N, Dechaud H, Pugeat M. Effects of diet and metformin administration on sex hormone-binding globulin, androgens and insulin in hirsute and obese women. J Clin Endocrinol Metab 1995;80:2057-62.
[25] Acbay O, Gundogdu S. Can metformin reduce insulin resistance in polycystic ovary syndrome? Fertil Steril 1996;65:946-9.
[26] Cassimirri F, Biscotti M, Gambineri A, Calzoni F, Eliana B, Pasquali R. Metformin improves insulin, body fat and distribution, androgens in obese women with and without the polycystic ovary syndrome [abstract]. Int J Obes 1997;21(suppl):S61.
[27] Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin sensitising agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovarian syndrome. J Clin Endocrinol Metab 1996;81:3299-306.
[28] Ehrmann D, Cavaghan M, Imperial J, Sturis J, Rosenfield R, Polonsky K. Effects of metformin on insulin secretion, insulin action and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1997;82:524-30.
[29] Nesder JE, Jakubowicz D. Decrease in ovarian cytochrome P450c17[Alpha] activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med 1996;335;617-23.
[30] Van der Spuy ZM, Dhansay R, Nugent FA. Adjuvant therapy with metformin to improve the therapeutic outcome in anovulatory hyperinsulinaemic women with polycystic ovary syndrome [abstract]. Hum Reprod 1996;11:167.
[31] Velazquez EM, Mendoza SG, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinaemia, insulin resistance, hyperandrogenaemia and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994;43:647-54.
[32] Velazquez EM, Acosta A, Mendoza SG. Menstrual cyclicity after metformin therapy in polycystic ovary syndrome. Obstet Gynecol 1997;90:392-5.
[33] Nestler JE, Jakubowicz D. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17[Alpha] activity and serum androgens. J Clin Endocrinol Metab 1997;82:4075-9.
RELATED ARTICLE: Summary points
It is evident that polycystic ovarian syndrome should no longer be considered a purely gynaecological disorder
Affected women seem m have subclinical insulin resistance and a form of the metabolic syndrome that manifests itself in early adult life with gynaecological symptoms
They may therefore gain particular benefit from early screening for cardiovascular risk factors, particularly glucose intolerance
Intervention with insulin sensitising agents, such as metformin, may play a major role in the future treatment of this condition, with the potential capacity to improve both endocrine and metabolic disturbances and reduce the risk of vascular disease
This approach may replace therapies used to treat individual components of polycystic ovarian syndrome such as hirsutism which may reflect a "downstream" feature of dais complex metabolic syndrome
RELATED ARTICLE: Diagnostic criteria of polycystic ovarian syndrome
Diagnosis is based on the presence of some or all of the common clinical features, confirmed by biochemical evidence of endocrine abnormality and exclusion of other possible aetiologies
Clinical features
* Oligomenorrhoea or dysfunctional uterine bleeding Anovulatory infertility
* Hirsutism or acne, or both
* Central obesity
Endocrine abnormalities
* Increased testosterone activity (often expressed as raised free androgen index)
* Elevated luteinising hormone concentration with normal follicle stimulating hormone concentration
* Insulin resistance with compensatory hyperinsulinaemia
RELATED ARTICLE: One hundred years ago
Hogarth's paintings at St. Bartolomew's Hospital
The paintings by Hogarth, to which a brief reference was made in the British Medical Journal of October 8th, ornament two of the walls of the grand staircase which leads to the Great Hall in the north wing of the hospital. The subjects are "The Good Samaritan" and "The Pool of Bethesda." They were painted gratuitously in 1736 by Hogarth, who was thereupon elected a life-governor of the charity. The pictures are well worthy of the careful treatment to which they have lately been subjected, for the painter in his autobiographical sketch says that they were his first efforts in the grand style. "Before I had done anything of much consequence in this walk," he says (thatis, the painting and engraving of modern moral subjects) "I entertained some hopes of succeeding in what the puffers in books call `the great literary style of history painting' so that without having done a stroke of this grand business before I quitted small portraits and familiar conversations, and with a smile at my own temerity commenced history painter, and on a great staircase at St. Bartholomew's Hospital painted two Scripture stories, `The Pool of Bethesda' and `The Good Samaritan, with figures 7 feet high." Both paintings were engraved in 1772, and impressions of the plates appear in Boydell's edition of Hogarth's works. (BMJ 1898;ii:1186)
University Department of Obstetrics and Gynaecology, Glasgow Royal Infirmary University NHS Trust, Glasgow G31 2ER
Zoe E C Hopkinson, clinical research fellow Richard Fleming, consultant grade biochemist
Ian A Greer, Muirhead professor of obstetrics and gynaecology
University Department of Clinical Biochemistry, Glasgow Royal Infirmary University NHS Trust
Naveed Sattar, spedalist registrar in clinical biochemistry
Correspondence to: Dr Hopkinson z.hopkinson@ clinmed.gla.ac.uk
BMJ 1998;317:329-32
COPYRIGHT 1998 British Medical Association
COPYRIGHT 2000 Gale Group