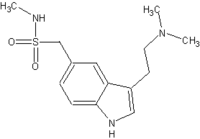
VANCOUVER, B.C. -- When migraine patients who need sumatriptan to abort their headaches fail to respond to it, they should try the injectable form, Seymour Diamond, M.D., said at the annual meeting of the American Headache Society.
In an open-label study in 43 patients, injectable sumatriptan--known to be the most effective of all the triptan drugs--alleviated 91% of 123 migraines in patients who had not responded to oral sumatriptan, Dr. Diamond of the Diamond Headache Clinic, Chicago, reported in a poster presentation.
When oral sumatriptan first came out, Dr. Diamond noted that two-thirds of the patients he switched to the drug eventually went back to the injectable form because they found it more effective. That observation prompted him to do the study, he said in an interview.
"I don't start with the injectable today," he said. "But I would go immediately to the injectable" in patients who don't respond to the oral formulation.
The study involved 43 patients with migraine refractory to oral sumatriptan who agreed to try the injectable form (6 mg subcutaneously) for three headaches. Four of the patients treated at least their first headache but not all three.
The drug was effective in alleviating pain and some of the symptoms in 91% of the first headaches treated, and provided complete relief from pain at 2 hours in 56% of the headaches. Responses for the subsequent headaches were roughly comparable.
No severe adverse events were reported by the trial participants, although one of the four patients who did not complete three doses dropped out because of multiple adverse effects.
The drug was effective even though the patients waited an average of 88 minutes after the onset of pain to inject themselves. Triptans tend to be less effective the longer a patient waits. Neurologists are becoming increasingly aware that many patients prescribed triptans tend to wait too long to use their medication, even when they have signs of an impending migraine.
BY TIMOTHY F. KIRN
Sacramento Bureau
COPYRIGHT 2005 International Medical News Group
COPYRIGHT 2005 Gale Group