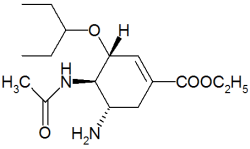
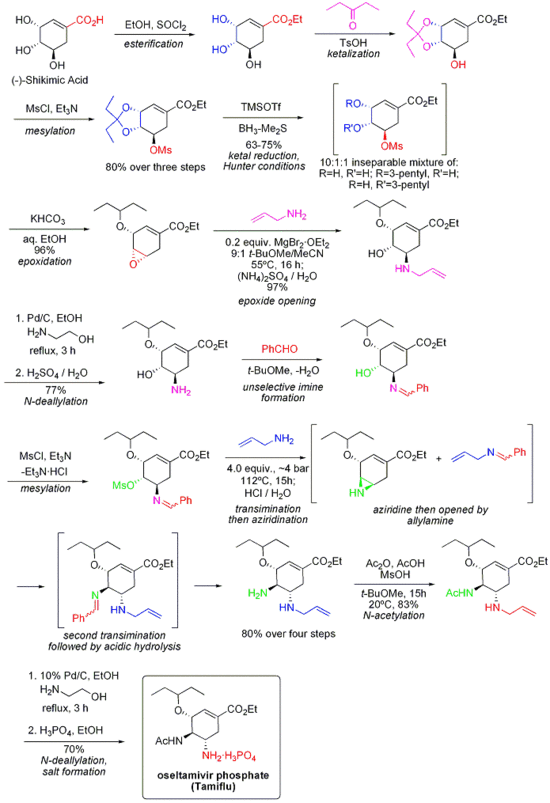
Tamiflu
Oseltamivir (pronounced ah sell TAH mih veer) is an antiviral drug used in the treatment and prophylaxis of both influenza A and influenza B. Like zanamivir, oseltamivir is a neuraminidase inhibitor, acting as a transition-state analogue inhibitor of influenza neuraminidase and thereby preventing new viruses from emerging from infected cells. Oseltamivir was the first orally active neuraminidase inhibitor commercially developed. more...
Oseltamivir is a prodrug (usually administered as phosphate); it is hydrolysed hepatically to the active metabolite, the free carboxylate of oseltamivir (GS4071).
Oseltamivir was developed by Gilead Sciences and is currently marketed by Hoffmann-La Roche (Roche) under the trade name Tamiflu®.
With increasing fears about the potential for a new influenza pandemic, oseltamivir has received substantial media attention. Production capacity is limited, and governments are stockpiling the drug.
Technical information
Indications and dosage
Roche recommendations in the United States
Tamiflu is available from Roche in 75mg capsules and as a powder for aqueous suspension of 12 mg/mL. According to prescription information by Roche for the United States, Tamiflu usage is indicated for both the treatment and prophylaxis of influenza at the following dosages.
- Tamiflu is indicated for the treatment of influenza in patients 1 year and older who have had symptoms for no more than two days. For influenza treatment, the standard dosage for patients 13 years and older is 75 mg twice daily for five days. Dosage for children is by weight.
- Tamiflu is indicated for prophylaxis of influenza either during a community outbreak or following close contact with an infected individual. Standard dosage is 75 mg once daily for patients aged 13 and older, which has been shown to be safe and effective for up to six weeks. Safety and efficacy for prophylaxis has not been established for patients under 13 years old.
The above treatment regimes are based upon studies of normal human influenza.
Dosage for avian flu
Peter Hobby (of the World Health Organization) has suggested that Vietnam should investigate and test a higher dosage and longer treatment with Tamiflu for patients with avian influenza. Doctors in Vietnam concur, noting that
t least in some patients with influenza A (H5N1) virus infection, treatment with the recommended dose of oseltamivir incompletely suppresses viral replication. Besides allowing the infection to proceed, such incomplete suppression provides opportunities for drug resistance to develop. (de Jong et al. 2005)
Co-administration with probenecid
It has been suggested that co-administration of oseltamivir with another drug called probenecid could dramatically extend the world's limited supply of oseltamivir. Probenecid reduces excretion of oseltamivir's active metabolite. 500 mg of probenecid given every six hours doubles oseltamivir's maximum blood concentration and also doubles the time that oseltamivir stays in the blood, multiplying a patient's overall exposure to the drug 2.5-fold. Probenecid was used in similar fashion during World War II to extend limited supplies of penicillin. The evidence for this interaction comes from a 2002 study by Roche (Hill et al. 2002), but was publicized only in October 2005 by a doctor who had reviewed the data (Butler 2005).
Read more at Wikipedia.org