Complying with AAP Lyme Disease Recommendations
To The Editor: American Family Physician published recommendations from the American Academy of Pediatrics (AAP) on the prevention and treatment of Lyme disease.(1) These recommendations appear straightforward. As usual, however, it is the fine print that trips us up. Here in the state of Maine, insurers have decided that the southern most county in the state is the only high-risk area; therefore, they will only pay for the Lyme vaccine for residents of that county.
A second problem is achieving compliance with yet another vaccine. Even worse is the problem of complying with a vaccine that consists of multiple doses.
A third problem is the price of the vaccine. I called my medical center pharmacy and the price for a single dose of the vaccine is $63.99. For three doses, $191.97 is a fairly pricey intervention. The suggestion in this practice guideline that the protective immunity is not known to last more than one year beyond dose 3 raises more questions about cost-effectiveness.
I found only one study evaluating cost-effectiveness of vaccination,(2) and it concluded that the mean cost of vaccination per case of Lyme disease averted is $4,466 based on the following assumptions: 0.80 probability of diagnosing and treating early Lyme disease, 0.005 probability of contracting Lyme disease and a vaccination cost of $50 per year.
I take issue with these assumptions as follows: I think 0.80 probability of diagnosing is an extremely high estimate; it is more likely to be 0.80 of those 50 to 60 percent of patients who have the erythema migrans rash of Lyme disease. Keeping the same attack rate as cited in the guidelines, and raising the vaccine cost per year to the $96, my patients would be charged a total of $383.94 (three doses over two years at $63.99 per dose). This is a fourfold reduction in benefit (or a cost of approximately $17,900 per case averted). I am omitting the nursing charge for vaccine administration, which would add another $10 per dose, because I assume there to be a slight mark up by my pharmacy.
REFERENCES
(1.) AAP issues recommendations on the prevention and treatment of Lyme disease [Practice Guidelines]. Am Fam Physician 2000;61:3463-4.
(2.) Meltzer MI, Dennis DT, Orloski KA. The cost effectiveness of vaccinating against Lyme disease. Emerg Infect Dis 1999;5:321-8.
Update on Treatment of Influenza A and B with Tamiflu
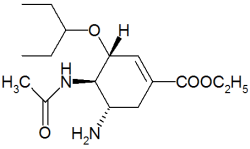
To The Editor: Since the article "Updated Treatment for Influenza A and B"(1) was accepted for publication, the U.S. Food and Drug Administration (FDA) has approved two new indications for oseltamivir (Tamiflu).(2) The FDA previously approved labeling for oseltamivir only for the treatment of uncomplicated influenza in adults.
The first new indication is the use of oseltamivir in the prevention of influenza in patients 13 years and older. Prophylactic treatment should be initiated within 48 hours of contact with an infected person. The recommended oral dose is 75 mg once daily for at least seven days, but it may be used for up to six weeks.
The second new indication is the treatment of acute illness from uncomplicated influenza in children one year or older who have been symptomatic for no longer than two days.(3,4) As with adults, treatment should be initiated within 48 hours of symptom onset. Twice daily dosing is based on body weight, and the drug will be supplied as a "tutti-frutti-tasting" suspension (12 mg per mL). The duration of treatment is five days.(3,4) The pediatric suspension formulation was expected to be released in mid-January. Adults who have difficulty swallowing capsules may also prefer the suspension.
Readers should note these important new changes since publication of our article.(1)
REFERENCES
(1.) Montalto NJ, Gum K, Ashley JV. Updated treatment for Influenza A and B. Am Fam Phys 2000; 62:2467-76.
(2.) www.fda.gov/bbs/topics/ANSWERS/ANS01056. html.
(3.) www.fda.gov/cder/drug/infopage/Tamiflu/
(4.) Tamiful (oseltamivir phosphate) capsules. Package insert. Nutley, NJ: Roche Laboratories, Inc. Revised December 2000.
Dr. Montalto receives an honorarium and is a member of the Speakers Bureau for Roche Laboratories and Glaxo Wellcome Inc.
Editor's Note: An updated text and table are available at www.aafp.org.
CASE REPORT
Sildenafil-Simvastatin Interaction: Possible Cause of Rhabdomyolysis?
TO THE EDITOR: I would like to report a case in which the concomitant use of sildenafil (Viagra) and simvastatin (Zocor) might have caused rhabdomyolysis. A 76-year-old man with a history of hypertension and hypercholesterolemia presented to the office with a complaint of erectile dysfunction. At that time, his medications included atenolol (Tenormin), 50 mg daily, and simvastatin, 10 mg daily (for at least three years' duration). Findings of his previous physical examination were within normal limits, including a metabolic panel and complete blood cell count that revealed no identifiable contraindications to sildenafil.(1)
The patient was prescribed sildenafil (50-mg tablets) to take two hours prior to sexual activity. Approximately 12 days after the patient began the trial of sildenafil, he presented to the clinic with a three-day history of unexplained, severe muscle aches (particularly in the lower extremities). The symptoms occurred eight to 10 hours after his use of sildenafil and resolved over the following three days.
Further questioning revealed no symptoms of viral illness or other identifiable causes of myalgias. He denied unusual or abnormal physical activity, trauma, burns, recent intramuscular injections or symptoms of coronary artery disease. He was completely asymptomatic at the time of his visit, and his physical examination did not reveal muscle tenderness or swelling.
Laboratory results showed a mild elevation of creatine phosphokinase (CPK) level of 406 IU per L. Transaminase levels, complete blood cell count, erythrocyte sedimentation rate and urinalysis were all within normal limits; however, there was a mild elevation of blood urea nitrogen (BUN) and an increase in creatinine and potassium levels from his baseline readings. Simvastatin was immediately discontinued. The patient had discontinued sildenafil after his single dose. He has been closely monitored and has done well since.
A review of the literature revealed no previous reports of drug interaction between simvastatin and sildenafil. Also, 3-hydroxy-3-methyl-glytaryl-coenzyme A (HMG-CoA) reductase inhibitors are not currently listed as a contraindication to the use of sildenafil.(2,3) However, pharmacologic data support the potential for drug interactions because both drugs are highly protein-bound and are metabolized by the liver via the cytochrome P450, 3A-4 pathway. It is possible that this competition causes a rise in the simvastatin level, increasing the risk for rhabdomyolysis.
I do not propose a cause and effect relationship but merely report an association with a possible biochemical explanation. Hopefully, further investigation will clarify this issue and provide information about the effect of these drugs on patients.
REFERENCES
(1.) Cheitlin MD, Hutter AM Jr, Brindis RG, Ganz P, Kaul S, Russell RO Jr, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol 1999;33:273-82.
(2.) Licht MR. Use of sildenafil (Viagra) in the treatment of erectile dysfunction. Compr Ther 1999;25:90-4.
(3.) Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404.
'Generally Safe' NSAIDs?
TO THE EDITOR: When will we ever learn? Physicians, patients and society have become so used to over-the-counter and prescription nonsteroidal anti-inflammatory drugs (NSAIDs) as an everyday fact of life that we have become numbed to the devastation they cause. In "Cyclooxygenase-2 Enzyme Inhibitors: Place in Therapy,"(1) the authors, in an otherwise excellent article, state: "Although generally safe, 'traditional' NSAIDs account for almost one fourth of all reported adverse drug events. Approximately 15 percent of NSAID users have gastrointestinal tract symptoms such as dyspepsia, heartburn, nausea or vomiting. Each year, 1 to 4 percent of NSAID users have serious gastrointestinal tract complications such as hemorrhage, with an estimated cost of $15,000 to $20,000 per hospitalization. An estimated 16,500 NSAID-related deaths occur annually among patients with osteoarthritis or rheumatoid arthritis. Medical costs of complications associated with NSAID use exceed $4 billion annually."(1)
Since when are 16,500 deaths, $4 billion dollars in complications and a 15 percent rate of gastrointestinal complications including gastrointestinal hemorrhage and other debilitating and potentially lethal side effects considered "generally safe"? The authors did not mention renal, liver and other associated toxicities. It's time we think of these medications as the dangerous and potentially lethal drugs they are. When we supervise a resident who casually prescribes ibuprofen to an elderly patient or when we ourselves feel pressed to find an easy answer to a throbbing back or head pain in a hypertensive patient we should think "danger" not "generally safe."
REFERENCE
(1.) Noble SL, King SD, Outade JI. Cyclooxygenase-2 enzyme inhibitors: place in therapy. Am Fam Physician 2000;61:3669-76.
IN REPLY: Dr. Rosen makes an excellent point. Perhaps the word "considered" should be inserted between the words "generally" and "safe." This article(1) tried to convey the significance of adverse events due to NSAID therapy by presenting the startling facts of NSAID-induced morbidity and mortality in that same paragraph.
In an earlier version of the article, the abstract did contain the sentence "NSAIDs can also adversely influence blood pressure control, the central nervous system, and cause renal complications." As the article went through medical and copy editing, this sentence was deleted. It was felt that family physicians are aware of the common adverse effects that NSAID therapy may cause. I appreciate your concern and would like to echo that NSAID therapy, although generally considered safe, indeed is not without significant risks.
REFERENCES
(1.) Noble SL, King SD, Outade JI. Cyclooxygenase-2 enzyme inhibitors: place in therapy. Am Fam Physician 2000;61:3669-76.
Send letters to Jay Siwek, M.D., Editor, American Family Physician, 11400 Tomahawk Creek Pkwy., Leawood, KS 66211-2672; fax: 913-906-6080; e-mail: afplet@ aafp.org. Please include your complete address, telephone number and fax number. Letters should be double-spaced, fewer than 500 words and limited to one table or figure and six references. Please submit a word count. Letters submitted for publication in AFP must not be submitted to any other publication. Possible conflicts of interest must be disclosed at time of submission. Submission of a letter constitutes transfer of copyright to the American Academy of Family Physicians. The editors may edit letters to meet style and space requirements.
COPYRIGHT 2001 American Academy of Family Physicians
COPYRIGHT 2001 Gale Group