Tay-Sachs disease is a fatal childhood degenerative disease of the central nervous system that is genetically transmitted and found primarily among Jews of Eastern European ancestry (Ashkenazim). Tay-Sachs is caused by the absence of a particular enzyme, hexosaminidase (hex-A). Without hex-A, fatty substances (lipids) build up in the brain and interfere with the functioning of the central nervous system. The disease is named for two doctors who were pioneers in identifying and describing it. In 1881, Warren Tay, a British ophthalmologist, first noted the red spot on the retina that is a distinctive mark of the disorder. In the United States, neurologist Bernard Sachs identified the cause of the illness and discovered its prevalence among Ashkenazic Jews in 1887.
Although the disease is already present in utero, the symptoms do not usually become apparent until the child is about five or six months old, at which point development is arrested and starts to regress. The child stops crawling and becomes unable to sit, push himself up, or perform other simple motor activities. He becomes easily startled, exhibiting an abnormal jerking motion (myoclonus), begins to lose his vision, and develops respiratory problems. By the second year of the disease, there is severe deterioration, including blindness, paralysis, deafness, seizures, and dementia, followed by death, usually between the ages of three and five.
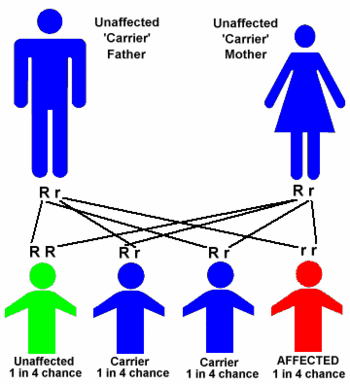
Tay-Sachs is transmitted by a recessive gene present in both parents, even though they do not have the disease themselves. For the disease to be passed on, both parents must be carriers. However, if even one parent is a carrier, the child has a 50% chance of also being a carrier. If both parents are carriers, their child has a 25% chance of being born with the disease. Although Tay-Sachs has been reported among other groups, including Amish and Italian Catholics, it is primarily found in Jews of Eastern European descent, in whom it is 100 times more frequent than in the general population. The average person has a one in 300 chance of being a carrier; an Ashkenazic Jew's chance of carrying the disease is about one in 30.
In the early 1970s, a pilot program was set up for genetic screening of potential Tay-Sachs carriers, who have visibly lower levels of the hex-A enzyme than non-carriers, although they have enough to prevent the lipid buildup that produces the disease. Testing centers were set up in Baltimore, Maryland, and an extensive educational campaign was launched in the Jewish community. More than 60,000 couples had their blood tested for levels of hex-A, and the screening identified a number of carriers of the disease. Since then, the International Tay-Sachs Disease Testing, Quality Control, and Data Collection Center has established and monitored testing centers throughout the world, and nearly one million young adults, both Jews and non-Jews, have been tested for the disease. The incidence of Tay-Sachs in the United States and Canada has plummeted from 60 new cases per year before 1970 to only three to five cases by the early 1990s--a 90% reduction. Couples who are carriers, and who decide to conceive, can learn in advance through amniocentesis whether their child will have the disease. More than 100 hospitals and clinics throughout the United States provide genetic screening for Tay-Sachs on an ongoing basis. Currently, approximately 62,000 persons worldwide are tested for the disease each year, 35,000 of them in the United States. Due to dramatic effectiveness of this testing program in reducing the incidence of the disease, it is considered a model for the application of genetic screening programs for other diseases.
One surprising discovery has been that the prevalence of Tay-Sachs disease among French-Canadian Catholics, which had received little attention until very recently, is roughly equal to that of Ashkenazic Jews. In a medical breakthrough of special significance for Catholic couples, who are unwilling to abort a pregnancy for religious reasons, a physician in Virginia has developed a procedure for screening an egg fertilized in a laboratory to determine if the child will be born with the disease. If it is found to be healthy, the pre-embryo can then be implanted in the mother's womb, and the parents can be assured of giving birth to a healthy baby.
At present there is no treatment for Tay-Sachs. Attempts to inject patients with the missing enzyme hex-A have failed because the substance was not absorbed by the brain after entering the bloodstream. Research on a cure is currently underway in the United States, Europe, and South Africa, with new hope provided by ongoing advances in molecular genetics and other relevant fields. The National Tay-Sachs and Allied Diseases Association (NTSAD), founded in 1958, promotes research, testing, and public education about the disease.
Further Reading
For Your Information
Books
- Brown, Fern G. Hereditary Diseases. New York: Franklin Watts, 1987.
Gale Encyclopedia of Childhood & Adolescence. Gale Research, 1998.