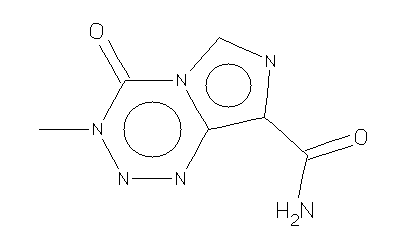
Some adult brain cancer patients now have another option for treating their disease.
The oral treatment temozolomide (Temodar) was approved by FDA in August to treat a form of brain cancer called anaplastic astrocytoma in patients who relapsed following initial treatment with radiation and chemotherapy.
Temozolomide was granted accelerated approval, a process FDA applies to some drugs for serious or life-threatening conditions. In a single study of the drug, tumors resistant to previous chemotherapy with two other drugs partially shrank and disappeared in 7 out of 54 patients. The most common side effects of temozolomide included headaches, nausea, vomiting, fatigue, and low blood counts.
At least 18,000 new cases of brain cancer are diagnosed each year in the United States, a figure which represents about 2 percent of all adult cancers. More than 50 percent of brain cancer cases are tumors that can cause severe disabilities such as motor dysfunction, seizures, and vision abnormalities.
As a condition of approval, FDA is requiring the manufacturer, ScheringPlough Corp., Madison, N.J., to further study the drug's effects on patients' survival or quality of life.
COPYRIGHT 1999 U.S. Government Printing Office
COPYRIGHT 2004 Gale Group