This inherited disorder causes serious lifelong problems. Find out what they are and how you can help.
Danny Caputo, 10 months old, is brought to the emergency department by his parents. His signs and symptoms include pallor, tachycardia, hepatosplenomegaly, poor feeding, and irritability. A complete blood cell count reveals a white blood cell count of 12,300/mm^sup 3^, hemoglobin of 6.1 grams/dl, and hematocrit of 16.4%. Both of Danny's parents are of Italian descent. They report that they know they carry the thalassemia trait in their blood and that several relatives have anemia.
The pediatric hematologist suspects β thalassemia major. He admits Danny for a transfusion of packed red blood cells (RBCs) and further diagnostic blood work.
In this article, we'll discuss the typical course of this disorder and explain what you can do to help a patient who has it.
Based in heredity
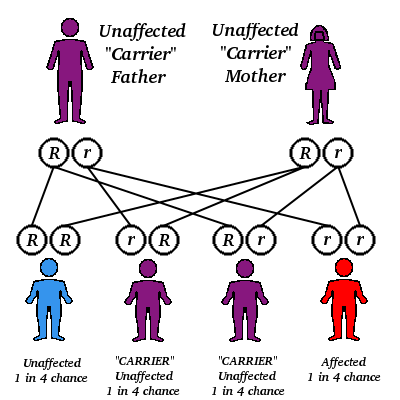
Thalassemia is an inherited blood disorder that most commonly affects people of Mediterranean, Middle Eastern, Indian, Asian, and Southeast Asian descent. Malaria is endemic in these areas, and the thalassemia trait, a genetic mutation, is thought to protect against malaria. When both parents have the trait (meaning they're carriers) and pass it to a child, he develops the disorder. Having the thalassemia trait generally doesn't cause symptoms, and a carrier may not know he has it until his child develops the disease. Each pregnancy in which both parents are carriers poses a 25% chance that the child will have thalassemia.
Different forms of thalassemia vary greatly in severity, but each affects the body's ability to produce a specific type of hemoglobin. Known as Cooley's anemia, β thalassemia major is the most severe form. It prevents or greatly reduces the body's ability to produce "adult" hemoglobin, Hb A.
The only available cure for β thalassemia major is a bone marrow transplant from a matched sibling. (See Who's a Candidate for Bone Marrow Transplant?) If this option isn't available, the patient needs lifelong blood transfusions to survive, generally starting before age 2.
Along with compatibility and infection risks, regular transfusions cause iron to accumulate in organs such as the heart, liver, and endocrine glands. Continued iron accumulation can cause organ failure and even death unless the iron is removed. (More on this shortly.)
Before transfusion therapy was available, children with β thalassemia major died in the first few years of life. Thanks to current treatments, people with the disease can survive into middle age.
Transfusions first
The pediatric hematologist and the health care team have discussed the risks and benefits of transfusion and gotten informed consent from Danny's parents. Now you need to assess their understanding and begin educating them about his illness. Through the course of this chronic disease, the patient and his family depend on your support, especially when the diagnosis is made, new therapies are added, and complications arise.
Gently explain to Danny's parents that his body can't produce enough normal hemoglobin to carry oxygen to his tissues, but transfusions can provide the hemoglobin he needs. Reassure them that with treatment, Danny's prognosis is good.
Explain that Danny will need regular blood counts and that when his hemoglobin level drops to a certain point (about 9.0 to 10.5 grams/dl, every 2 to 4 weeks), he'll receive a transfusion of RBCs with most of the white blood cells removed. (Leukoreduction reduces the risk of adverse reactions.) This regimen will maximize his growth and function and minimize toxic iron accumulation in his tissues.
Chelation therapy lies ahead
When Danny reaches age 3 or 4, he'll start chelation therapy to bind and excrete excess iron from his body. In children of this age with β thalassemia major, blood ferritin levels generally exceed 1,500 nanograms/ml. Chelation therapy consists of subcutaneous infusions of deferoxamine (Desferal) lasting 8 to 12 hours every night.
When a child starts chelation therapy, the parents must learn to prepare the medication, select and rotate subcutaneous administration sites, and recognize adverse drug reactions and signs of infection. As the therapy successfully removes the accumulated iron, the child's urine will appear orange, red, or tea colored.
Complications of deferoxamine therapy may include swelling and itching at the administration site, blurry vision, and high-frequency hearing loss, possibly with tinnitus. Some young children have developed genu valgus (knock-knees) because chelation removes other metals from the body. Annual hearing and vision exams and long-bone X-rays are recommended to screen for these problems. If a problem is identified, the patient will discontinue the drug until the adverse reaction resolves, then restart it at a lower dose.
A psychosocial problem sometimes associated with chelation therapy, especially among adolescents and young adults, is the need for a "holiday." The patient may become so tired of self-administering the nightly subcutaneous infusions that he stops, although he may not admit to his action. A rising ferritin or liver iron value may signal a break from therapy.
If your patient or his parents lose motivation for traditional chelation therapy, try to negotiate a workable alternative. Other options include placement of a percutaneous intravenous line or indwelling catheter, hospitalization at regular intervals (such as on weekends) for administration of high doses, and subcutaneous bolus injections.
Sometimes, splenectomy
In the years ahead, Danny and his parents will have to learn about the possible need for splenectomy. If a patient with β thalassemia major doesn't receive transfusions, splenomegaly develops as his body tries to compensate for severe anemia by enlisting all possible sites of RBC production. Extramedullary hematopoiesis and blood sequestering in the spleen can cause significant splenomegaly, but it typically resolves when transfusion therapy is started.
Sometimes, splenomegaly persists even after the child undergoes chronic transfusion therapy. Splenectomy may be warranted if his annual blood requirement is greater than 200 ml/kg/year, if he needs more than 700 ml per transfusion, or if he develops persistent thrombocytopenia. The benefits of splenectomy must be weighed against the increased risks of sepsis and pulmonary hypertension due to thrombocytosis.
Bony deformities too
Untreated β thalassemia major can cause bone deformities such as a prominent forehead and flattened nasal bridge. These changes reflect years of bone marrow expansion as the body attempts to increase hematopoiesis. More subtle changes include paraspinous masses and osteoporosis, possibly as early as age 5. Once the patient is regularly receiving transfusions, the progression of bony deformities should stop. Adequate intake of calcium and vitamin D and appropriate therapy with growth hormone will delay the development of osteoporosis.
Once a fatal illness, β thalassemia major has become a chronic disease thanks to transfusions and effective chelation therapy. Plenty of education and support over the years will help Danny and his family manage the multiple demands of his illness and improve his quality of life.
SELECTED REFERENCES
Brittenham, G.: "Disorders of Iron Metabolism: Iron Deficiency and Overload," in Hemalology: Basic Principles and Practice, 3rd edition, R. Hoffman, et al. (eds). New York, N.Y., Churchill Livingstone, 2000.
Hagege, I., et al.: "Long-Term Administration of High-Dose Deferoxamine 2 Days per Week in Thalassemic Patients," European Journal of Haematology. 67(4):230-231, October 2001.
Kattamis, A., et al.: "Variations of Ferritin Levels over a Period of 15 Years as a Compliance Chelation Index in Thalassemic Patients," American Journal of Hematology. 68(4):221-224, December 2001.
Lucarelli, G., et al.: "The Cure of the Thalassemia with Bone Marrow Transplantation," Bone Marrow Transplantation. 28(Suppl., 1):S11-S13, August 2001.
Schrier, S.: "Pathophysiology of Thalassemia," Current Opinion in Hematology. 9(2):123-126, March 2002.
Tiosano, D., and Hochberg, Z.: "Endocrine Complications of Thalassemia," Journal of Endocnnological Investigation. 24(9)716-723, October 2001.
By Marie Boorman Martin, RN; Dru Foote, RN, PNP, MS; and Susan Carson, RN, CPNP, MSN
Marie Boorman Martin is a thalassemia nurse at Children's Hospital of Philadelphia (Pa.), Dru Foote is a thalassemia nurse practitioner at Children's Hospital of Oakland (Calif.), and Susan Carson is a thalassemia nurse practitioner at Children's Hospital of Los Angeles (Calif.).
Copyright Springhouse Corporation Oct 2004
Provided by ProQuest Information and Learning Company. All rights Reserved