Practice recommendations
* Self-monitoring of blood glucose is an integral component of diabetes therapy and should always be included in the management plan (SOR: C).
* Medical nutrition therapy should be individualized, preferably by a registered dietitian familiar with diabetes (SOR: B).
* A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR: B).
* When a monotherapy fails, combine drugs with different mechanisms of action to achieve an additive effect (SOR: A).
* The combination of sulfonylurea and metformin has proven effective in many studies. One showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A).
Glycemic control in diabetes begins with a patient's adherence to several non-pharmacologic measures. Without such a commitment, success in controlling the disease will be difficult to achieve, and otherwise appropriate drug therapy will be hindered.
Most antidiabetic agents comparably reduce glycosylated hemoglobin ([A.sub.1c]) levels. However, a particular agent may be preferred depending on a patient's characteristics. And some circumstances call for combination therapy. This article reviews the advantages and disadvantages of the many pharmacologic treatments for glucose control and hyperglycemia in type 2 diabetes.
* BENEFITS OF DIABETES CONTROL
The benefits of diabetes control are detailed in this issue of THE JOURNAL OF FAMILY PRACTICE ("Strategies to reduce complications in type 2 diabetes," pages 366-374). For every percentage-point reduction in hemoglobin [A.sub.lc], it is possible to achieve a 22% to 35% reduction in microvascular complications. (1,2) Cardiovascular disease can be reduced in patients with diabetes by treating hypertension (3,4) and hyperlipidemia, prescribing aspirin therapy, using angiotensin-converting enzyme (ACE) inhibitors, and with smoking cessation. (5,6)
* TARGETS FOR GLYCEMIC CONTROL
The American Diabetes Association's (ADA) recommended targets for glycemic control are a preprandial blood glucose level of 80-120 mg/dL, a bedtime blood glucose level of 100-140 mg/dL, and a hemoglobin [A.sub.1c] level of <7% (with a level of >8% requiring additional measures). Hemoglobin [A.sub.1c] is the best determinant of glycemic exposure, and its mean value is a nationally recognized indicator of how well diabetes is being managed. (7) The American College of Endocrinology has adopted a more aggressive approach by designating an [A.sub.1c] level of 6.5% as both a target and action level. (8)
Self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is an integral component of diabetes therapy (strength of recommendation [SOR]: C) and should always be included in the management plan (SOR: C). The optimal frequency and timing of SMBG for type 2 diabetes is not known, but they should be sufficient to facilitate reaching glucose goals. The [A.sub.1c] test should be performed at least semi-annually for patients with stable glycemic control, and quarterly for patients not meeting glycemic goals or those who are changing therapy. [A.sub.1c] levels and mean plasma glucose levels can be approximately correlated (Table 1). (7)
* NONPHARMACOLOGIC THERAPY
Nonpharmacologic measures remain the cornerstone of managing type 2 diabetes. Hyperglycemia adversely and reversibly affects both insulin resistance and insulin secretion. Improvement in glycemic control can occur through dietary modification and regular exercise.
A recent meta-analysis of randomized controlled trials of diabetes patient education observed a net decrease in Hb[A.sub.1c] of 0.32% in intervention groups vs control. (9) Interventions that included a face-to-face delivery, cognitive refraining teaching method, and exercise content were more likely to improve glycemic control.
Education
Lifestyle changes involving diet, exercise, and usually weight loss are key to effective management of diabetes. If patients are to change their behavior, they must be given detailed training. (6) Self-management also necessitates that patients engage in problem solving. This requires that each aspect of the management plan is understood and agreed upon by the patient and providers, and that the goals and treatment plan are individualized and reasonable.
Diet: recommend soluble fiber, reduce calories
Medical nutrition therapy should be individualized and preferably provided by a registered dietitian familiar with diabetes (SOR: B). The goals of nutrition therapy, according to the ADA, are to attain recommended body weight and prevent or reverse obesity. The means of achieving these goals are nutrition assessment and modification of nutrient intake and lifestyle through healthy food choices and physical activity. (7)
A high intake of dietary fiber (particularly the soluble type) above the level recommended by the ADA improves glycemic control, decreases hyperinsulinemia, and lowers plasma lipid concentrations. (10)
Hypocaloric diets cause glucose plasma levels to fall, in some cases to a normal level with a weight loss of even 5 to 10 pounds. (7,11) Hypoglycemic medications are of course most effective in nonobese persons. But effectiveness is also improved if weight that is gained can be limited. Despite the clear benefit of weight loss, only a few patients are able to attain and maintain substantial weight loss. Maintenance of a reduced or elevated body weight is associated with compensatory changes in energy expenditure, which oppose the maintenance of body weight that is different from the usual weight. (13) Part of the individualization of therapy is respect of personal and cultural preferences, lifestyles, and financial considerations.
[FIGURE 1 OMITTED]
Physical activity: a little goes a long way
A regular physical activity program is recommended for all patients with diabetes who are capable of participating (SOR: B). (7) It improves blood glucose control, reduces cardiovascular risk factors, aids weight loss, and enhances well being/ A recently published prospective cohort study showed that walking at least 2 hours a week was associated with a 39% lower all-cause mortality (hazard rate ratio [HRR], 0.61; 95% CI, 0.48-0.78) and a 34% lower cardiovascular mortality (HRR, 0.66; 95% CI, 0.45-0.96) across a diverse spectrum of adults with diabetes. The NNT (to prevent 1 death per year) is 61 for patients who walk at least 2 hours/week. (14)
In prescribing a physical activity plan for a patient, consider cardiovascular disease risk factors or complications to minimize the risk of untoward events. Micro- and macrovascular disease are of course prevalent among persons with diabetes, often resulting in functional limitations that make exercise more difficult.
Other priorities
Other recommended components of care include daily aspirin use, foot care exams, tobacco cessation, pneumococcal and influenza vaccinations, and an annual dilated retinal exam.
* PHARMACOLOGIC THERAPY
The coexisting defects in type 2 diabetes mellitus are as follows:
* resistance to insulin action in muscle
* defective pancreatic insulin secretion
* unrestrained hepatic glucose production, aggravated by increased lipolysis in adipose tissue.
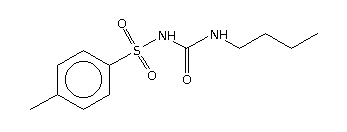
Drug therapy is aimed at each of these defects, and also at reducing carbohydrate absorption in the small intestine (Figure 1). As far as antihyperglycemic effect is concerned, no one category of antidiabetic agent is favored over another. (15) Except for nateglinide and [alpha]-glucosidase inhibitors (AGIs), each of the drug categories leads to a similar reduction in [A.sub.1c]. (16) However, patient characteristics may lead to selection of a particular agent. Table 2 summarizes oral treatment options, their relative advantages and costs.
[FIGURE 1 OMITTED]
Sulfonylureas
Sulfonylureas directly increase insulin secretion by binding to the sulfonylurea receptor on pancreatic beta cells; they provide a relatively quick onset of action. First-generation sulfonylureas (eg, tolbutamide, chlorpropamide) and second-generation sulfonylureas (eg, glyburide, glipizide, glimepiride) are equivalent in their maximum hypoglycemic effect. (17)
Second-generation agents are used more commonly than first-generation. They all contain the sulfonylurea moiety, but different chemical substitutions in the basic molecule change pharmacokinetics, resulting in different durations of action. (17) Second-generation agents are probably safer than first-generation drugs, being less likely to cause hyponatremia, disulfiram-like reactions, or prolonged hypoglycemia. (18)
At maximal doses, sulfonylureas lower [A.sub.1c] levels by 1-2 percentage points and fasting plasma glucose concentrations by 60-70 mg/dL; (15) however, the glucose lowering effect typically plateaus after half the maximal recommended dose is reached. Sulfonylureas have no consistent effect on dyslipidemia. In UK Prospective Diabetes Study (UKPDS) 33, though improved glycemic control with sulfonylureas (or insulin) led to a 25% reduction in microvascular endpoints (mostly less retinal photocoagulation) (P<.01), sulfonylureas (or insulin) did not significantly reduce death or all-cause mortality compared with diet treatment. (2)
Adverse effects. The primary adverse effects of sulfonylureas are weight gain and hypoglycemia. In UKPDS 33, weight gain at 10 years was 2.6 kg (99% confidence interval [CI], 1.6-3.6) with chlorpropamide and 1.7 kg (99% CI, 0.7-2.7) with glyburide, compared with patients receiving diet therapy (each P<.001). (2) In the same study, the rate of major hypoglycemic episodes (third-party help or medical intervention necessary) while on therapy was 0.4%/year for chlorpropamide and 0.6%/year for glyburide, compared with 0.1%/year for diet.
Glyburide and chlorpropamide have active metabolites with renal elimination, and they should therefore be used with caution in patients with renal insufficiency. In 1971, the University Group Diabetes Project (UGDP) observed a twofold increase in the rate of cardiovascular death among patients receiving tolbutamide compared with those receiving insulin or placebo. (18) This led to a decades long debate on the validity of this conclusion. (19) More recently, UKPDS 33 did not demonstrate any increased cardiovascular mortality among patients receiving glyburide or chlorpropamide, and has largely negated this earlier concern. (2)
Cost. Sulfonylureas are the least expensive oral agents used to treat type 2 diabetes.
Non-sulfonylurea secretagogues
Like sulfonylureas, the non-sulfonylurea secretagogues (non-SU), repaglinide and nateglinide, stimulate beta cells to increase insulin secretion. However, the non-SU agents mediate their action through a different, adjacent site on the "sulfonylurea receptor." Comparatively, the non-SU agents have a faster onset of action (20 minutes), shorter half-life (about 1.0-1.5 hours), and greater effects on postprandial glucose excursions than do sulfonylureas. (20) In contrast to the sulfonylureas, the extent of insulin release with non-SU agents is glucose dependent, and therefore they may have less risk of hypoglycemia several hours after meals. (15)
Repaglinide lowers the [A.sub.1c] level by 1.7-1.9 percentage points, similar in efficacy to sulfonylureas. Nateglinide appears somewhat less efficacious and lowers [A.sub.1c] by 0.6-1.0 percentage points. (15) Nateglinide was significantly less effective than glyburide at lowering [A.sub.1c] levels and the fasting plasma glucose in one 24-week study. Non-SUs added to sulfonylureas produce no additional benefit in glycemic control. The effect of non-SUs on microvascular or macrovascular endpoints is unknown.
Adverse effects. Hypoglycemia is the primary adverse effect of non-SUs. Confirmed hypoglycemia (plasma glucose <60 mg/dL) was observed in 2.4% of patients taking nateglinide compared with 0.4% of those receiving placebo. Mild or moderate hypoglycemia occurred in 16% of repaglinide patients, 20% of glyburide patients, and 19% of glipizide patients in one-year comparative studies. Further comparative studies are needed to determine if non-SUs produce significantly less hypoglycemia and weight gain than sulfonylureas.
Cost. Non-SUs must be dosed 3 times daily at the start of meals. One relative disadvantage is their increased cost compared with sulfonylureas.
Biguanides
The only biguanide marketed in the US is metformin. Its primary action is to inhibit hepatic glucose production and, to a much lesser extent, enhance insulin sensitivity in peripheral tissues. (21) Metformin does not stimulate insulin secretion and does not cause hypoglycemia when used as monotherapy, but it can potentiate hypoglycemia in combination with insulin or insulin secretagogues.
Metformin is similar in efficacy to the sulfonylureas. It lowers [A.sub.1c] by 1.5-2.0 percentage points and fasting plasma glucose by 60-80 mg/dL. Its antihyperglycemic efficacy is independent of patient age, duration of diabetes, or BMI. (22)
In the UKPDS 34 study, a subgroup of obese patients was randomized to receive intensive control (group 1, metformin; group 2, a sulfonylurea or insulin) or conventional diet therapy (group 3). Despite a similar reduction in the [A.sub.1c] level between the 2 intensive-treatment groups, patients treated with metformin had a 32% reduction for any diabetes-related endpoint (95% CI, 13-47; P=.002), 43% fewer diabetes-related deaths (95% CI, 9-63; P=.017), and a 36% reduction in all cause mortality, compared with the diet therapy group (95% CI, 9-55; P=.011). (23)
Metformin also showed significant benefit when compared with patients receiving sulfonylurea or insulin (group 2). The absolute risk of any diabetes endpoint was 29.8 vs. 40.1 (events per 1000 patient-years; P=.0034), all-cause mortality (13.5 vs 18.9; P=.021), and stroke (3.3 vs 6.2; P=.032), respectively, for metformin vs sulfonylurea or insulin (group 2). Thus, metformin is the only oral hypoglycemic agent proven to reduce macrovascular risk in overweight patients with type 2 diabetes. For perspective, in overweight patients, metformin significantly reduced all-cause mortality (NNT per year=141; 95% CI, 115-183; P=.011), and any diabetes-related outcome (NNT per year=74; 95% CI, 63-90; P=.0023), compared with diet alone. (23,24)
Metformin induces weight loss (2-3 kg), preferentially involving adipose tissue in obese patients with type 2 diabetes over 4 to 6 months. (22,25) In UKPDS 34, weight gain was similar among those treated with metformin and diet (approximately 2 kg); weight gain over 10 years was less with metformin, however, than with sulfonylurea (approximately 4 kg) or insulin (approximately 6 kg). (23) Metformin also significantly improved levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides when compared with glyburide or placebo. (22)
Risk of lactic acidosis. Lactic acidosis associated with metformin is a rare but serious adverse event, with an estimated prevalence of 3 cases per 100,000. (26) The product labeling notes most of these cases have occurred among patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion. Absolute contraindications include renal disease (serum creatinine [greater than or equal to] 1.5 mg/dL [males] and [greater than or equal to] 1.4 mg/dL [females]), congestive heart failure requiring pharmacological treatment, and acute or chronic metabolic acidosis. It should also be discontinued at the time of radiologic studies using intravascular iodinated contrast materials.
Additional "precautionary conditions" include age [greater than or equal to] 80 years (unless measurement of creatinine clearance demonstrates that renal function is not reduced), hepatic disease, cationic drug use, conditions associated with hypoxia (eg, chronic obstructive pulmonary disease [COPD], acute myocardial infarction, dehydration, sepsis), excessive alcohol intake, and surgery, until patient's oral intake is resumed.
Is the risk overstated? Despite these extensive precautions, published studies show that metformin is commonly prescribed to patients with absolute contraindications. (27,28) One recent study observed that 11.2% of Medicare beneficiaries hospitalized with congestive heart failure and concomitant diabetes were treated with metformin. (28) In the absence of advanced renal dysfunction, metformin rarely accumulates in the body, (29) and accumulation of metformin is rarely reported as a cause of lactic acidosis. (30,31) Rather, tissue hypoxia acts as a trigger in most cases. Metformin should therefore be discontinued whenever tissue hypoxia is suspected. (31)
A recent systematic review and meta analysis found no evidence that metformin was associated with an increased risk of lactic acidosis if the drug was prescribed under study conditions, taking into account contraindications. (32) Refinement and clarification of the risk for lactic acidosis in these various populations is needed, to ensure optimal patient safety and to further assess this highly effective medication.
Common adverse effects associated with metformin are diarrhea and nausea, which can be minimized by administering the drug with meals and slowly titrating the dose, or perhaps by using the extended-release formulation.
Thiazolidinediones
Thiazolidinediones (TZDs) include rosiglitazone and pioglitazone. These agents, like metformin, do not increase insulin secretion but depend on the presence of insulin for their activity. TZDs are agonists at peroxisome-proliferator-activated receptor gamma (PPAR-[gamma]) receptors in peripheral tissues such as skeletal muscle, where they increase glucose uptake. (15) Thus, their predominant effect is to decrease insulin resistance.
TZDs have similar antihyperglycemic efficacy as sulfonylureas or metformin. They decrease [A.sub.1c] levels by 0.6-1.9 percentage points and lower fasting plasma glucose levels by 50-80 mg/dL. (15) They have a slower onset of action compared with other hypoglycemic drugs, and intervals of 3 to 4 weeks should be allowed between doses before increasing the dosage. TZDs also have favorable effects on lipid levels: HDL concentrations increase and triglyceride concentrations decrease with their use. (33) It is not known whether they decrease macrovascular or microvascular complications, although such studies are underway.
Adverse effects. TZDs are typically well tolerated, though weight gain of 1-3 kg, edema (4%-5%) and anemia (1%-2%) can occur. Weight gain and edema are more pronounced when TZDs are used in combination with insulin. Anemia is likely due to increased plasma volume rather than any significant hematological effect.
Due to adverse events related to volume expansion, TZDs are not recommended for patients with New York Heart Association class III or IV heart failure. A recent consensus statement from the American Heart Association and the ADA stresses that before administering TZD treatment, the physician should explore the possible presence of cardiac disease, use of other drugs that cause fluid retention, and the pathogenesis of any existing edema or dyspnea. (34)
Although troglitazone was removed from the market due to its association with hepatocellular injury, pioglitazone and rosiglitazone are not as convincingly associated with liver injury. (15) In preapproval clinical studies, less than 0.5% of patients treated with rosiglitzone and pioglitazone had elevations in alanine transaminase (ALT) >3 times the upper limit of normal.
The incidence of hepatitis or acute liver failure from troglitazone was compared with rosiglitazone, pioglitazone, metformin, and glyburide, by analysis of spontaneously reported adverse events to the Food and Drug Administration (FDA) MEDWATCH database during the first 15 months of marketing of each drug. (35,36) The incidence of hepatitis per million prescriptions was 21.5, 14.7, 9.4, 2.9, and 4.1, respectively, while the incidence of acute liver failure per 100,000 prescriptions was 4.6, 0.9, 0.8, 0.2, and 0. It appears that postmarketing data support preclinical studies, in that the incidence of acute liver failure is an order of magnitude higher for troglitazone vs. other TZDs. (35) However, the FDA recommends avoiding their use in patients with baseline ALT levels >2.5 times the upper limit of normal. The FDA recently reduced the recommended frequency for ALT monitoring for pioglitazone (and is currently considering the same for rosiglitazone). Serum ALT is recommended prior to initiation and then periodically thereafter.
Cost. TZDs are expensive relative to other hypoglycemic agents.
[alpha]-glucosidase inhibitors
The [alpha]-glucosidase inhibitors (AGIs), acarbose and miglitol, act through competitive, reversible inhibition of membrane-bound intestinal [alpha]-glucosidase, which hydrolyzes complex carbohydrates to glucose and other monosaccharides. This inhibition delays glucose absorption and decreases postprandial hyperglycemia. (37) Thus, they have a nonsystemic mechanism of action.
These agents cause a modest reduction in the [A.sub.1c] level (0.5-1.0 percentage points) and are thus less effective than sulfonylureas, metformin, or TZDs. They do not reduce fasting plasma glucose levels, but reduce postprandial hyperglycemia by 50 mg/dL. (38) No long-term studies have evaluated whether AGIs reduce diabetes-related macrovascular or microvascular outcomes.
Adverse effects. While AGIs are virtually free of serious toxicities, patient tolerability can be a problem due to adverse gastrointestinal effects. In indirect comparisons from placebo-controlled trials, patients treated with miglitol and acarbose commonly reported abdominal pain (11.7%, 19%), diarrhea (28.7%, 31%), and flatulence (41.5%, 74%), respectively. Systemic accumulation of AGIs has been shown to increase in proportion to the degree of renal insufficiency, and their use is not recommended for patients with serum creatinine >2.0 mg/dL. However, whether such patients are at greater risk of any toxicity is unknown. Acarbose at doses above 100 mg 3 times daily has been associated with elevated serum transaminase levels; however, this risk appears negligible at standard doses.
Insulin
Insulin is the oldest therapy for diabetes, and it has no upper dose limit. (39) It increases insulin levels and can reduce [A.sub.1c] levels by 1.5 to 2.5 percentage points. Though half of diabetes patients need insulin eventually for optimal control, historically it has been introduced late in the disease process unless patients have severe hyperglycemia (fasting blood sugar >350 mg/dL) or ketonuria. (38) However, it is effective in gaining initial control, decreasing gluconeogenesis and increasing glucose uptake. Disadvantages are weight gain, hypoglycemia, and patient reluctance to give injections.
When insulin is indicated. Patients who exhibit persistent hyperglycemia despite oral hypoglycemic therapy may stop the oral drug(s) and begin insulin. By combining insulin with oral therapy, lower insulin doses may be used to achieve desired control vs using insulin alone. (40) For some patients a basal supplement of insulin may be sufficient and can be given as a single dose at bedtime, without an oral hypoglycemic drug. (41)
Insulin regimens. Various insulin regimens are available: very rapid acting (lispro and aspart), rapid acting (regular), intermediate acting (isophane insulin [NPH] and lente) and very long acting (ultralente and glargine). Glargine insulin (Lantus) has more predictable absorption than NPH, lente, and ultralente. Lantus, compared with NPH, has been associated with less nocturnal and postprandial hypoglycemia. (38,42,43) This is consistent with the peakless and longer duration of glargine compared with NPH. (44) A recent randomized controlled trial demonstrated that morning insulin glargine lowered [A.sub.1c] levels more than a bedtime dose of NPH (-1.24 vs -0.84; 95% CI, 0.23%-0.58%) or a bedtime dose of glargine (-1.24 vs -0.96%; 95% CI, 0.11%-0.46%). (45) Glargine's only relative disadvantage is increased cost.
Combination products. Combination insulin options are 70 NPH/30 regular, 50 NPH/50 regular, and 75 lispro protamine/25 lispro. Many combinations of insulin regimens have been used successfully. The typical range of insulin needed for monotherapy is 0.4-1 U/kg/d. Once-daily injection of intermediate acting or long acting insulins at bedtime or before breakfast, once-daily or twice-daily combinations of intermediate and rapid acting insulins, and more complex regimens have been used to good effect.
Using prandial insulin at each meal with separate basal insulin adds flexibility to meal times and doses administered. (43) With multiple-dose intensive insulin therapy, a basal dose suppresses hepatic glucose output and the bolus doses enhance postprandial glucose uptake. This intensive insulin treatment reduces mortality among critically ill patients in surgical intensive care units and for those with acute myocardial infarction. (46,47) An algorithm for using progressive therapy in diabetes mellitus is shown in Figure 2. (48)
[FIGURE 2 OMITTED]
* COMBINATION THERAPY
Over time glycemic control becomes more difficult, even with maximum monotherapy for patients with healthy lifestyles. It was shown in UKPDS 49 that monotherapy with sulfonylurea, metformin, or insulin eventually fails in most cases--by 3 years after diagnosis, about 50% of patients need more than monotherapy; 75% by 9 years. (49) In UKPDS 33, the median [A.sub.1c] level increased steadily over 10 years with both conventional therapy and intensive therapy (Figure 3). (2)
[FIGURE 3 OMITTED]
Several options are available when monotherapy fails. Based on expert opinion, the principle is to combine drugs with different mechanisms of action to achieve an additive effect for glycemic control. Combination products may simplify the treatment regimen and improve adherence. In many instances, they may also cost less. (50)
Successful combinations. The combination of sulfonylurea and metformin has proven effective in many studies. (22,51,52) One study showed that initial treatment with glyburide/metformin improved glycemic control better than either glyburide or metformin monotherapy (SOR: A). (53,54) The addition of the non-SU secretagogues repaglinide and nateglinide to metformin significantly improved glycemic control, with repaglinide showing superiority over nateglinide. (55) A TZD added to a sulfonylurea has also significantly improved [A.sub.1c] and fasting blood sugar results. (56) Patients whose diabetes was inadequately controlled with diet alone or diet plus a sulfonylurea showed improvement with the addition of the AGI miglitol, compared with addition of placebo. (57) The AGI acarbose has shown to be an effective addition to diet, metformin, sulfonylurea, and insulin. (58) A TZD added to metformin has also been shown to improve glycemic control. (59) A non-SU added to patients inadequately controlled with a TZD has also been effective. (60)
The early addition of insulin when maximal sulfonylurea therapy is inadequate has been effective. (61-63) When introducing insulin, a nighttime regimen of NPH or glargine, 10 units at bedtime, is an appropriate dose (SOR: C). This is easier and less costly than often assumed, and helps improve glycemic control. (64) Most patients require combination therapy as their disease progresses. (39)
* IMPROVING OUTCOMES
Cumulative survey data reveal a wide gap between guideline recommendations and the care patients receive. (65) One study showed that physicians initiated treatment changes only after the [A.sub.1c] level had reached 9.0% or higher instead of the 8.0% level recommended by ADA. (66) How can the quality of management be improved?
In private practices and institutions, many interventions have been shown to improve outcomes in diabetes mellitus. Education measures work, and they include chart audits, reminder cards, pharmacist collaboration, flow sheets, and nursing initiatives. (67,68) Effective disease-management programs have also used clinical guidelines, outcomes reporting, coverage of glucose meters and strips, and the support of clinical leadership. (69)
Computerized systems that track patients and recommended laboratory tests have improved screening rates and glycemic and blood pressure control. (70) Monitoring patients' readiness to change has allowed targeted education to improve [A.sub.1c] levels. (71) Continuity of care has also improved the quality of disease control by increasing adherence to recommended tests and exams. (72)
ACKNOWLEGMENTS
The authors thank Marie Hamer, RN, for her continuous diabetes quality improvement efforts and Jean Camarata for her editorial and reference acquisition assistance.
REFERENCES
(1.) Vinik AI, Vinik E. Prevention of the complications of diabetes. Am J Manag Care 2003; 9 suppl:S63-S80.
(2.) UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837-853.
(3.) Arauz-Pacheco C, Parrott MA, Raskin P; American Diabetes Association. Treatment of hypertension in adults with diabetes. Diabetes Care 2004; 27 (suppl):S65-67.
(4.) UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703-713.
(5.) Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 2002; 287:2570-2581.
(6.) Kendall DM, Bergenstal RM. Comprehensive management of patients with type 2 diabetes: establishing priorities of care. Am J Manag Care 2001; 7 (suppl):S327-S343.
(7.) American Diabetes Association. Standards of medical care in Diabetes. Diabetes Care 2004; 27(suppl):15-35.
(8.) Peterson KA. Diabetes management in the primary care setting: summary. Am J Med 2002; 113(suppl 6A) :36S-40S.
(9.) Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: A meta-analysis and meta-regression. Patient Educ Couns 2004; 52:97-105.
(10.) Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000; 342:1392-1398.
(11.) Hadden DR, Montgomery DAD, Skelly RJ, et al. Maturity onset diabetes mellitus: response to intensive dietary management. Br Med J 1975; 3:276-278.
(12.) Niskenen LK, Uusitupa MI, Surlund, H, et al. Five-year follow-up study on plasma insulin levels in newly diagnosed NIDDM patients and nondiabetic subjects. Diabetes Care 1009; 13:41-48.
(13.) Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332:621-628.
(14.) Gregg EW, Gerzoff RB, Caspersen CJ, et al. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med 2003; 163:1440-1447.
(15.) Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287:360-372.
(16.) Holmboe ES. Oral antihyperglycemic therapy for type 2 diabetes: clinical apparatus. JAMA 2002; 287:373-376.
(17.) Rang HP, Dale MM, Ritter JM, Moore PK. The endocrine pancreas and the control of blood glucose. In: Pharmacology. 5th ed. London: Churchill-Livingstone/Elsevier Science; 2003:380-394.
(18.) Davis SN, Granner DK. Insulin, oral hypoglycemic agents, and the pharmacology of the endocrine pancreas. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001:1679-1714.
(19.) Goldner MG, Knatterud GL, Prout TE. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. 3. Clinical implications of UGDP results. JAMA 1971; 218:1400-1410.
(20.) Hollander P, Schwartz SL, Gatlin MR, et al. Importance of early insulin secretion: comparison of nateglinide and glyburide in previously diet-treated patients with type 2 diabetes. Diabetes Care 2001; 24:983-988.
(21.) Inzucchi SE, Maggs DG, Spollett GR, et al. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 1998; 338:867-872.
(22.) DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995; 333:541-549.
(23.) UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352:854-865.
(24.) Shaughnessy AF, Slawson DC. What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ 2003;327:266.
(25.) Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 1995; 333:550-554.
(26.) Brown JB, Pedula MS, Barzilay J, et al. Lactic acidosis rates in type 2 diabetes. Diabetes Care 1998; 21:1659-1663.
(27.) Calabrese AT, Coley KC, DaPos SV, Swanson D, Rao RH. Evaluation of prescribing practices: risk of lactic acidosis with metformin therapy. Arch Intern Med 2002; 162:434-437.
(28.) Masoudi FA, Wang Y, Inzucchi SE, et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA 2003; 290:81-85.
(29.) Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 1996; 30:359-371.
(30.) Lalau JD, Lacroix C, De Cagny B, Fournier A. Metformin-associated lactic acidosis in diabetic patients with acute renal failure. A critical analysis of its pathogenesis and prognosis. Nephrol Dial Transplant 1994; 9 (suppl 4):126-129.
(31.) Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ 2003; 3:131-132.
(32.) Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus: systematic review and metaanalysis. Arch Intern Med 2003; 63:2594-2602.
(33.) Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathiesen AL, Schnieder RL. Pioglitazone hydrochloride monotherapy improves giycemic control in the treatment of patients with type 2 diabetes: a 6 month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000; 23:1605-1611.
(34.) Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation 2003; 108:2941-2948.
(35.) Tolman KG, Chandramouli J. Hepatotoxicity of the thiazolidinediones. Clin Liver Dis 2003; 7:369-379.
(36.) Zawadzki JK, Green L. Graham BJ. Thioglitazone-associated 15-month post-marketing hepatotoxicity. Poster abstract. FDA Science Forum. Available at: vm.cfsan.fda.gov/~frf/forum02/a187ab4.htm. Accessed on February 25, 2004.
(37.) Lebowitz HE. a-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev 1998; 6:132-145.
(38.) Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc 2003; 78:459-467.
(39.) Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med 2002; 347:1342-1349.
(40.) Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A metaanalysis. Diabetes Care 1992; 15:953-959.
(41.) Cusi K, Cunningham GR, Comstock JP. Safety and efficacy of normalizing fasting glucose with bedtime NPH insulin alone in NIDDM. Diabetes Care 1995; 18:843-851.
(42.) White JR, Davis SN, Cooppan R, et al. Clarifying the role of insulin in type 2 diabetes management. Clinical Diabetes 2003;1:14-21.
(43.) DeWitt DE, Hirsch IR. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA 2003; 289:2254-2264.
(44.) Yki-Jarvinen H, Dressler A, Ziemen M; HOE 901/3002 Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care 2000; 23:1130-1136.
(45.) Fritsche A, Schweitzer MA, Haring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003; 138:952-959.
(46.) van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345:1359-1367.
(47.) Malmberg K, Norhammar A, Wedel H, Ryden L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999; 99:2626-2632.
(48.) Zimmerman BR. Therapy for type 2 diabetes mellitus. In: Medical Management of Type 2 Diabetes. 4th ed. Alexandria, Va: American Diabetes Association; 1998.
(49.) Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus. Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281:2005-2012.
(50.) Leichter SB, Thomas S. combination medications in diabetes care: an opportunity that merits more attention. Clin Diabetes 2003; 21:175-178.
(51.) Hermann LS, Schersten B, Bitzen P, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care 1994; 17:1100-1109.
(52.) Jeppesen J, Zhou M, Chen Y, Reaven G. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care 1994; 17:1093-1099.
(53.) Garber AJ, Larsen J, Schneider SH, et al. Simultaneous glyburide/metformin therapy is superior to component monotherapy as an initial pharmacological treatment for type 2 diabetes. Diabetes Obes Metab 2002; 4:201-208.
(54.) Riddle M. Combining sulfonylureas and other oral agents. Am J Med 2000; 108(suppl 6A):15S-22S.
(55.) Raskin P, Klaff L, McGill J, et al. Efficacy and safety of combination therapy: repaglinide plus metformin versus nateglinide plus metformin. Diabetes Care 2003; 26:2063-2068.
(56.) Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med 2001; 111:10-17.
(57.) Johnston PS, Feig PU, Coniff RF, Krol A, Davidson JA, Haffner SM. Long-term titrated-dose a-glucosidase inhibition in non-insulin-requiring Hispanic NIDDM patients. Diabetes Care 1998; 21:409-415.
(58.) Chiasson J, Josse RG, Hunt JA, et al. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med 1994; 121:928-935.
(59.) Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA 2000; 283:1695-1702.
(60.) Fonseca V, Grunberger G, Gupta S, Shen S, Foley JE. Addition of nateglinide to rosiglitazone monotherapy suppresses mealtime hyperglycemia and improves overall glycemic control. Diabetes Care 2003; 26:1685-1690.
(61.) Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR; UKPDS. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002; 25:330-336.
(62.) Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Arch Intern Med 2003; 163:1781-1782.
(63.) Westphal SA, Palumbo PJ. Insulin and oral hypoglycemic agents should not be used in combination in the treatment of type 2 diabetes. Arch Intern Med 2003; 163:1783-1785.
(64.) DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA 2003; 289:2265-2269.
(65.) Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med 2002; 136:565-574.
(66.) Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care 2003; 9:213-217.
(67.) Sutherland JE, Hoehns JD, O'Donnell B, Wiblin RT. Diabetes management quality improvement in a family practice residency program. J Am Board Fam Pract 2001; 14:243-251.
(68.) De Grauw W, van Gerwen W, van de Lisdonk EH, van den Hoogen HJ, van den Bosch WJ, van Weel C. Outcomes of audit-enhanced monitoring of patients with type 2 diabetes. J Fam Pract 2002; 51:459-464.
(69.) Sidorov J, Gabbay R, Harris R, et al. Disease management for diabetes mellitus: impact on hemoglobin A1c. Am J Manag Care 2000; 6:1217-1226.
(70.) Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care 1999; 5:1299-1307.
(71.) Peterson K, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Faro Pract 2002; 15:266-270.
(72.) Parchman ML, Burge SK. Continuity and quality of care in type 2 diabetes: a Residency Research Network at South Texas study. J Fam Pract 2002; 51:619-624.
(73.) Fagan TC, Deedwania PC. The cardiovascular dysmetabolic syndrome. Am J Med 1998; 105(suppl):77S-82S.
(74.) Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344:1343-1350.
(75.) Meigs JB. The metabolic syndrome. BMJ 2003; 327:61-62.
Metabolic syndrome
A group of metabolic abnormalities that increase cardiovascular risk has been recognized since 1988 and has been given many names--Syndrome X, insulin resistance syndrome, dysmetabolic syndrome, The Deadly Quartet. (73) The National Cholesterol Education Program Adult Treatment Panel III recently recodified this syndrome as shown below. The principles for diet and exercise discussed in this article also apply to the goals of reducing obesity and physical inactivity in the metabolic syndrome, and preliminary data suggest a reduction in the risk for type 2 diabetes (NNT per year=27; P=.0001 (74) and for cardiovascular disease. (75)
John E. Sutherland, MD Northeast Iowa Family Practice Residency Program, Waterloo; University of Iowa College of Medicine, Iowa City
James D. Hoehns, PharmD Northeast Iowa Family Practice Residency Program, Waterloo; University of Iowa College of Pharmacy, Iowa City
Corresponding author: John E. Sutherland, MD, Northeast Iowa Family Practice Residency Program, University of Iowa College of Medicine, 2055 Kimball Avenue, Waterloo, Iowa 50702. E-mail: jsutherl@neimef.org.
COPYRIGHT 2004 Dowden Health Media, Inc.
COPYRIGHT 2004 Gale Group