The problem of reduction in the rate and extent of dissolution of formulations containing gelatin in the outer layer (in hard and soft gelatin capsules and sugar-coated tablets), which appears after gelatin products are stored is a matter of concern for pharmaceutical manufacturers as well as regulatory agencies. Previously, the authors investigated several questions related to gelatin stability, including a rapid method for evaluating the possibility of reduction in the dissolution rates of gelatin-based formulations, the evaluation of gelatins for cross-linking potential, the influence of bloom strength and type of gelatin on cross-linking, and the use of the film approach for the stabilization of gelatin preparations against cross-linking (1-4). The problem also has been reviewed (5,6).
In this study, the authors investigate the role of functional groups in the drugs on the cross-linking of gelatin induced by formaldehyde. Because gelatin cross-linking is accelerated by formaldehyde, glutaraldehydes, glyceraldehydes, hydrogen peroxide, benzene, sulfonic acid, and guanidine hydrochloride, and is inhibited by amino acids such as glycine, lysine, phenylalanine, glutamine, hydroxylamine hydrochloride, p-aminobenzoic acid, semicarbazide, piperazide hydrate, pyridine, piperidine, and glycerine, drugs with similar functional groups were expected to show a parallel behavior (7,8).
The study was carried out on 25 randomly selected drugs, that were incorporated into soft gel films. Soft film was selected as a test platform because drugs are present at a high concentration in soluble or suspended state in soft gel capsules, thus offering ample opportunity for drug-gelatin interaction. The films were subjected to dissolution before and after exposure to formaldehyde. The results of the study highlight the differential effect of drugs on gelatin cross-linking.
Materials and methods
Gelatin of bloom strength 100 was provided by Gelita (Sioux City, Iowa). Glycerol was purchased from SISCO Research Laboratories Pvt. Ltd (Mumbai, India), Methyl paraben and citric acid were purchased from Loba Chemie (Mumbai, India), and propyl paraben, glycine, and formaldehyde solution (37-41%) were purchased from S.D. Fine Chemicals Ltd (Boisar, India).
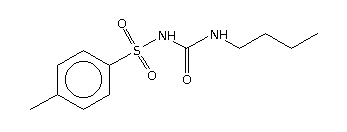
The drugs were procured from bulk drug or formulation manufacturers, as follows: atenolol, frusemide, nimesulide, ethambutol, and isoniazid (Panacea Biotec Limited, Lalru, India); ranitidine hydrochloride, losartan, and atorvastatin (Ranbaxy Laboratories Limited, Toansa, India); rofecoxib and celecoxib (Unichem Laboratories Limited, Baddi, India); mefenamic acid (Blue Cross Laboratories Limited, Nashik, India); cetrizine (UCB Pharma Limited, Mumbai, India); metronidazole (Arti Drugs Limited, Sion E, India); ampicillin trihydrate and amoxicillin trihydrate (DSM Antiinfectives, Toansa, India); zidovudine (Ind-Swift Laboratories Limited, Dera Bassi, India); dapsone (Atul Limited, Atul, India); acyclovir (Medicorp Technologies India Limited, Chennai, India); fexofenadine (Dabur Research Foundation, Sahibabad, India); haloperidol (Avik Pharmaceutical Limited, Mumbai, India); tolbutamide (Quantum Drugs and Chemicals, Madurai, India); and dextromethorphan (Wochardt Limited, Mumbai, India).
Gelatin mass was prepared on a rotary film evaporator (B-480, Buchi Labortechnik AG, Flawil, Germany) and matured in a precision water bath (MV, Julabo Labortechnik GmbH, Seelbach, Germany). The films were prepared using a laboratory coating device (SV-M-101301, Mathis, Zurich, Switzerland). Film thickness was measured using a digital screw gauge (Mitutoyo Corp., Kangawa, Japan). Dissolution studies were conducted using a magnetic heating and stirring device (Remi Equipments, Mumbai, India). A photostability chamber (KBF240, WTB Binder, Tuttlingen, Germany) was used to expose the films to accelerated conditions. Weighings were conducted on a precision balance (AG135, Mettler Toledo, Greifensee, Switzerland).
Preparation of soft gelatin films and rings. Table I lists the formulas used for the preparation of soft gelatin films with and without drugs and a stabilizer. The procedure for preparing soft gelatin films and rings was the same as described in a previous article from the authors (4). Initially, the films were casted to a constant thickness of 6 mm and kept in the refrigerator for 12 h for hardening. The hardened films then were cut into small rings of 11 mm i.d. and 14 mm o.d., keeping the weight constant at 35 mg by cutting the outer edge of the ring.
Exposure of the rings to formaldehyde. The experimental setup for formaldehyde exposure was the same as previously reported by the authors (2). The overall exposure scheme is given in Figure 1. The drug-containing rings were initially exposed to 78 [micro]g formaldehyde for 12 h and if the rings dissolved in < 1 h, then another set of rings containing the same drug was exposed to a higher concentration of formaldehyde (117.5 [micro]g). However, if the rings exposed to 78 [micro]g for 12 h failed to dissolve in 1 h, then separate sets of rings of the same batch were subjected to shorter exposure periods of 2, 4, 6, 8, and 10 h. In this case, another set of films was tested after the addition of a stabilizer combination (4). Dissolution studies also were conducted on the rings that contained the drugs but were not exposed to formaldehyde, to serve as controls.
[FIGURE 1 OMITTED]
Dissolution studies. Dissolution studies were conducted in 200 mL aqueous medium in an open circular glass dish (10 cm in diameter and 4 cm in height) (2). The films were placed in duplicate in a wire mesh basket that was dipped as high as half the height of the dissolution medium in the dish. The temperature was maintained at 37 [+ or -] 0.5[degrees]C and stirring was carried out with a magnetic stirrer. The end point was the time taken for complete disappearance of the film as a result of dissolution. It was possible to record the end point within [+ or -] 3 min for the replicate samples.
Some of the films dissolved in <60 min and others took longer. Films were considered to pass the dissolution test when the time taken for complete dissolution was <60 min. The films were considered to fail the test if time taken for complete dissolution was >60 min.
Results and discussion
To evaluate their influence on the cross-linking of gelatin, drugs were directly incorporated into the gelatin mass before the preparation of the films and rings. Although this method did not exactly replicate the conditions of the soft gelatin capsules in which drugs are encapsulated as a solution or suspension, the film model was still considered suitable for delineating the differentiating nature of the influence of drugs on gelatin cross-linking. The drugs included in the study were procured randomly, without considering whether their formulations (solutions or suspensions) were marketed in soft gel capsules or not. This was done to meet the basic objective of determining the specific role of functional groups in drug-gelatin interaction.
The rings were exposed to formaldehyde and dissolved in an aqueous solution. Initial exposure was to 78 [micro]g formaldehyde and the rings that dissolved completely were exposed to a larger amount of formaldehyde (117.5 [micro]g). This procedure confirmed whether the resistance to cross-linking shown by the drug, if any, existed even when higher amounts of a catalyst were present. Studies involving the addition of glycine and citric acid to rings that failed the dissolution test after exposure to 78 [micro]g formaldehyde for 2 h also were conducted to check whether this previously reported stabilizer combination worked even in the presence of the drugs in the gelatin films (4).
Dissolution results. All unexposed soft gelatin rings with or without drug dissolved completely within 6-15 min. The rings without drug failed in dissolution tests after exposure to 78 [micro]g formaldehyde for 2 h.
The data for drug-containing rings appear in Table II. As evident, there was a wide variation in results, with some of the drugs actually preventing cross-linking (pass results after exposure to 117.5 [micro]g formaldehyde for various time periods), others showing delay (pass results after exposure to 78 [micro]g formaldehyde for various time periods), and still others having no (or catalytic) action (fail result even after exposure to 78 [micro]g formaldehyde for 2 h).
The results for drug-containing rings that failed the dissolution test even after exposure to 78 [micro]g formaldehyde for 2 h and then tested with a stabilizer combination, appear in Table III (4). All of the films completely dissolved in <16 min in the presence of drugs, suggesting that the stabilizer combination was able to prevent cross-linking.
Classification of drugs into various categories. Based on the results in Table II, the drugs were classified into various categories, as described in Figure 2. The drugs for which the dissolution time of the films was significantly lower than the dissolution time of the film without drug, were classified as cross-linking inhibitors (Category I) (e.g., isoniazid, rantidine hydrochloride, etc.). Some of the drugs, including dapsone, losartan, furosemide, etc., did not inhibit cross-linking significantly, and only prolonged the time required to induce cross-linking. These drugs were classified as cross-linking prolongers (Category II). All of the remaining drugs that showed a failed dissolution test after exposure to 78 [micro]g formaldehyde for 2 h were classified as Category III.
[FIGURE 2 OMITTED]
Order of capability of drugs to resist or inhibit cross-linking. The drugs in Categories I and II in Figure 2 are displayed in decreasing order of their ability to inhibit or delay cross-linking. Because no such ordering was possible in Category III, those drugs are listed alphabetically.
Role of functional groups. The structures of the drugs were screened for various types of functional groups present, and the functional groups for each category are listed in Table IV. Drugs in the first category of cross-linking inhibitors all contain one or more primary amino groups (e.g., isoniazid and acyclovir) or one or more secondary amino groups (e.g., ethambutol). Primary amino derivatives (e.g., glycine) are known to interact with aldehydic impurities and behave as cross-linking stabilizers, and the results of this study indicate that drugs containing similar primary and even secondary amino groups also can possess gelatin-stabilizing properties (8).
The drugs falling in the second category contain either a primary amino group (dapsone, frusemide, celecoxib), one or more secondary amino groups (atorvastatin, mefenamic acid), or a combination of primary, secondary, and/or tertiary amino groups (atenolol, ampicillin trihydrate, amoxiclnin trihydrate), along with functional groups such as carboxyl, carbonyl, aldehyde, keto, and sulfonamide, which are known to accelerate cross-linking (7, 8). The fact that drugs in this category resist cross-linking by formaldehyde suggests that the overall basicity of the amino groups in the molecules is stronger overall than the combined effect of oppositely acting (catalytic) functional groups.
Drugs in the third category are devoid of amino groups (metronidazole), contain one or more secondary/tertiary amino groups (dothiopin, diclycomine), and/or have one or more of functional groups that induce cross-linking (nimesulide, rofecoxib, tolbutamide). It is because of their structure that the drugs in this category showed complete cross-linking on exposure to 78 [micro]g formaldehyde for 2 h.
Necessity of stabilizers. The study shows that drugs with primary and secondary amines as major functional groups can neutralize aldehyde-induced gelatin cross-linking and, therefore, when present in formulations, may not need the addition of stabilizers. For the second category, it may be safe to add the stabilizers, but the addition of stabilizers is a must for the third category of drugs.
Catalytic action of drugs. There is a definite possibility that drugs with predominant functional groups such as carboxyl, carbonyl, aldehyde, keto, and sulfonamide may show an acceleration of cross-linking similar to the effect shown by formaldehyde. However, the present study was not able to differentiate between non-active drugs and drugs with catalytic activity within Category III because all of the drugs in this category showed complete dissolution failure, similar to that shown by gelatin rings containing no drug.
Correlation of results with the observations reported in the literature. In a few studies reported in the literature, the cross-linking problem was observed with drugs contained in the soft gelatin capsules. Those drugs are gemfibrozil and nifedipine (9, 10). Examination of the structure of these molecules revealed that both drugs have predominant catalytic groups, hence there is a likelihood that drugs themselves also had a role in promoting the observed extent of cross-linking (11). Thus, the results of this study coincide with the observations reported in the literature.
Conclusion
The study highlights the influence of drug molecules on the extent and rate of dissolution of gelatin formulations. The study indicates that the extent of cross-linking depends on the nature of the functional groups present on the drug molecules, and the influence may range from cross-linking inhibition to no effect or even promotion of cross-linking (which is suggested by but not proven in this study). It is suggested that based on the chemical structure of a drug to be encapsulated in gelatin formulations, an assessment can be made of its likely influence on the gelatin shell. Accordingly, a decision can be made regarding the addition of stabilizers in either the film or the fill (4,8).
The study also raises the possibility of developing predictive relationships between drug structure and the extent of cross-linking of gelatin films, especially among drugs belonging to Categories I and II. The authors plan to take up these studies in the future.
References
(1.) S. Singh, R. Manikandan, and S. Singh, "Stability Testing for Gelatin-Based Formulations: Rapidly Evaluating the Possibility of a Reduction in Dissolution Rates," Pharm. Technol. 24 (5), 58 72 (2000).
(2.) K. Venugopa] and S. Singh, "Evaluation of Gelatins for Cross-Linking Potential," Charm. Technol. Drug Delivery Supplement 32-37 (2001).
(3.) K.V.R. Ran and S. Singh, "Sensitivity of Gelatin Raw Materials to Cross-Linking: The Influence of Bloom Strength, Type, and Source," Pharm. Technol. 26 (12), 42-46 (2002).
(4.) K.V.R. Rao, S.P. Pakhale, and S. Singh, "A Film Approach for the Stabilization of Gelatin Preparations Against Cross-Linking, Pharm. Technol. 27 (4), 54q53 (2003).
(5.) S. Singh et al., "Alteration in Dissolution Characteristics of Gelatin-Containing Formulations: A Review of the Problem, Test Solutions, and Solutions," Pharm. Technol. 26 (4), 36-58 (2002).
(6.) S. Singh and S. P. Pakhale, "Gelatin-Containing Formulations: Changes in Dissolution Characteristics," Encyclopedia of Pharm. Technol. Online. (2003).
(7.) C.A. Marks, D. Tourtenotte, and A. Andux, "A Phenomenon of Gelatin Insolubility," Food. Technol. 22 1433-1436 (1968).
(8.) T.A. Adesunloye and P.E. Stach, "Effect of Glycine/Citric Acid on the Dissolution Stability of t lard Gelatin Capsules," Drug Dev. Ind. Pharm. 24 (6), 493-500 (1998).
(9.) L. Chafetz et al., "Decrease in the Rate of Capsule Dissolution Due to Formaldehyde from Polysorbate 80 Autooxidation," J. Pharm. Sci. 73 (8), 1186-1187 (1984).
(10.) C.B. Bottom, M. Clark, and J.T. Carstensen, "Dissolution Testing of Soft Shell Capsules--Acetaminophen and Nifedipine," J. Pharm. Sci. 86 (9), 1057-1061 (1997).
(11.) The Merck Index (Merck & Co., Rahaway, NJ, 11th ed., 1989), pp. 626, 936.
Please rate this article.
On the Reader Service Card, circle a number.
348 Very useful and informative
349 Somewhat useful and informative
350 Not useful or informative
Your feedback is important to us.
Deepti Gholap and Saranjit Singh *
* To whom all correspondence should be addressed.
Deepti Gholap is a postgraduate student and Saranjit Singh, PhD, is a professor and head of the department of pharmaceutical analysis at the National Institute of Pharmaceutical Education and Research, Sector 67, S.A.S. Nagar 160 062, India, tel. +91 172 2214682, fax +91 172 2214692, ssingh@niper.ac.in.
COPYRIGHT 2004 Advanstar Communications, Inc.
COPYRIGHT 2004 Gale Group