The steroids trenbolone acetate (TbA) and melengestrol acetate (MGA) are licensed as growth promoters for farm animals in several meat-exporting countries. Although many studies have explored their safety for both animals and consumers, little is known about their fate after excretion by the animal. Our study aimed to determine the residues and degradation of trenbolone and MGA in solid dung, liquid manure, and soil. In animal experiments lasting 8 weeks, cattle were treated with TbA and MGA. Solid dung and, in case of trenbolone, liquid manure were collected and spread on maize fields after 4.5 and 5.5 months of storage, respectively. Determination of the hormone residues in all samples included extraction, clean-up (solid-phase extraction), separation of metabolites and interfering substances by HPLC (RP-18), and quantification by sensitive enzyme immunoassay. Procedures were validated by mass spectrometry (MS) methods. During storage of liquid manure the level of trenbolone decreased from 1,700 to 1,100 pg/g (17[alpha]-isomer), corresponding to a half-life of 267 days. Before storage, the concentrations in the dung hill ranged from 5 to 75 ng/g TbOH and from 0.3 to 8 ng/g MGA. After storage, levels up to 10 ng/g trenbolone, and 6 ng/g MGA were detected. In the soil samples trenbolone was traceable up to 8 weeks after fertilization, and MGA was detected even until the end of the cultivation period. The results show that these substances should be investigated further concerning their potential endocrine-disrupting activity in agricultural ecosystems. Key words: degradation, dung, growth promoter, manure, melengestrol acetate, soil, trenbolone. Environ Health Perspect 109:1145-1151(2001). [Online 2 November 2001] http://ehpnet1.niehs.nih.gov/docs/2001/109p1145-1151schiffer/abstract.html
For several years we have known that sex hormones excreted via human and/or animal feces can exhibit endocrine-disrupting activity; for example, estrogens present in chicken manure caused hyperestrogenism when fed to cattle (1). Natural and synthetic estrogens such as estradiol-17[beta] and ethinylestradiol-17[alpha] were frequently detected in lower nanogram per liter ranges in discharges of sewage treatment plants, caused by their incomplete removal during passage through the sewage treatment plants (2). Exposure of fish to sewage treatment plant effluents increased plasma levels of vitellogenin, a protein synthesized by the liver of oviparous fish in response to estradiol stimulation (3). Public concern focuses especially on the synthetic estrogen and progestin components of oral contraceptives, which have high physiologic activity at low doses. Compared with the natural hormones, they show a relatively greater stability in aqueous media (4) and a greater resistance to microbial degradation (5). These properties pose the potential for accumulation and persistence in the environment. It can be presumed that other structurally related xenobiotic hormones that are used for veterinary treatment show a similar behavior.
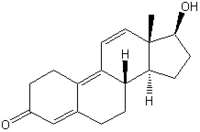
The synthetic steroids trenbolone acetate [TbA (17[beta]-acetoxyestra-4,9,11-triene-3-one); Figure 1] and melengestrol acetate [MGA (17[alpha]-acetoxy-6-methyl- 16-methylene-pregna-4,6,-diene-3,20-dione); Figure 2] are licensed as growth promoters for farm animals in the United States and Canada. TbA is administered as a subcutaneous implant either alone or in combination with an estrogenic compound. The anabolic effect of TbA, which is 8-10 times stronger than that of testosterone propionate (6), is based on androgenic and antiglucocorticoid activity (7,8). After its release from the depot into the blood circulation, TbA is hydrolyzed to the active trenbolone-17[beta] (TbOH-17[beta]). In the heifer, only one major metabolic route occurs, oxidation of TbOH-17[beta] to trendione (TbO), followed by reduction to TbOH-17[alpha] (Figure 1). The epimerization strongly decreases the compound's biologic efficacy; the anabolic potency of TbOH-17[alpha] is only about 5% of that of TbOH-17[beta] (9), and the affinity to the recombinant human androgen receptor (rhAR) is reduced to about 4% (10).
[FIGURES 1-2 OMITTED]
Melengestrol acetate (MGA), an orally active gestagen, can be used for estrus synchronization and/or induction in cattle (11). It is also marketed as a feed additive for feedlot heifers to improve feed efficiency and rate of weight gain. The administered daily dose of 0.5 mg per cow allows ovarian follicular development while inhibiting estrus and ovulation (12). MGA exerts both progestional and glucocorticoid activity (11). Its progestional activity was about 125 times greater than that of progesterone as measured by estrus cycle inhibition in cattle (13); anti-inflammatory assays in rats showed that its glucocorticoid activity was comparable with that of hydrocortisone (14). The anabolic mode of action of MGA is assumed to be due to stimulation of the ovarian synthesis of endogenous estradiol (15). Androgenic side effects are probably not of concern because a recent study has shown that the binding affinity of MGA to the rhAR is only about 1% of testosterone and 0.3% of dihydrotestosterone (10).
Although many studies have been performed on the safety of TbA and MGA for both animals and consumers (11,13,16), little is known about their fate after excretion by animals. It is possible that these substances and/or their metabolites accumulate in soil or find their way into surface or even ground water via dung or manure. The intention of our studies was to determine the residues and degradation of TbOH and MGA, respectively, in solid dung, liquid manure, and soil.
Material and Methods
Animal Experiments, Manure Collection, and Field Experiments
All animals used in our research have been treated according to the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the guiding principles in the Guide for the Care and Use of Laboratory Animals, National Institutes of Health (17).
Study I: Degradation of TbOH in liquid manure. We implanted 41 cattle (Holstein Friesian) with commercially available anabolic preparations containing TbA. The total amount of TbA applied to the animals was 3,340 mg. The liquid manure was collected in a manure collection canal and pumped into the cylindric manure storage pit, open at the top, every 2 weeks. In the collection canal the material was not homogeneous, whereas in the manure storage a stirring propeller achieved good homogeneity before sampling. The manure was stored under anaerobic conditions. After the end of the animal experiments the total volume of the manure in the storage was about 170 [m.sup.3] and contained all animal excrement, the stablecleaning water, and atmospheric precipitations (rain, snow) that also reached the storage. The estimated mass of excrement was 100 tons.
Samples of liquid manure were taken every second week from the collection canal (to survey the conditions immediately after the manure formation), every 2 or 4 weeks from the manure storage tank, and before spreading on the fields. A small fraction of the total amount was spread on an experimental field in November after the end of the animal experiments. The majority was used for fertilization in spring after 5.5 months of storage. We took samples of the stored manure every month. All samples were stored at -25 [degrees] C.
Study II: Stability of TbOH in solid dung. We implanted 12 Holstein Friesian heifers with commercially available TbA preparations. The total amount of TbA applied in the experiment was 5,600 mg. For cleaning of the stables, the excrement of the animals was removed in a traditional procedure with the help of straw, and a dung hill was erected. After the end of the animal experiments, the dung hill contained the excrement of the 12 animals from day 31 before treatment to day 56 after treatment. The estimated total volume of the dung hill was 40 [m.sup.3]; the estimated mass of excrement was 20 tons.
After finishing the animal experiments, we took samples of solid dung from four different locations of the dung hill, representing different regions (top, middle, bottom, and liquid effluent). In November the solid dung was transferred to a sealed storage ground. Mixing of the dung hill during transportation was inevitable. After 4.5 months of storage, samples were again taken from different regions (top, middle, and bottom). All samples were kept at -25 [degrees] C.
Study III: Residues of melengestrol acetate in feces and solid dung. We treated 13 Holstein Friesian heifers with MGA, served as feed premix that was prepared from reference material (ICN Biomedicals, Eschwege, Germany) at the Institute of Animal Nutrition at the Technical University of Munich-Weihenstephan, Germany. The total amount of MGA applied in the experiment was 840 mg. The excrement was removed with the help of straw similar to study II, but the dung hill was erected automatically by pressing the fresh dung from the bottom of the dung plate into the dung hill. After the end of the animal experiments, the dung hill contained about 20 tons of excrement in an estimated total volume of 60 [m.sup.3].
Samples of feces were taken twice per week from each animal. Sampling and storage of solid dung were performed analogously to study II.
Studies IV and V: Steroid residues and stability in soil. At our experimental farm the liquid manure and solid dung from the hormone treatment experiments were used to fertilize fields on which maize was cultivated according to good agricultural practices.
Liquid manure containing TbOH was spread on one section of the fields in November (fresh manure) and on another section in March (stored manure). Solid dung from studies II and III was brought out also in March. Soil samples were collected from three representative locations of each experimental section of the fields, some immediately after fertilization, and regularly every month (first 3 months) or every second month, respectively, until the end of the cultivation period (i.e., ploughing of the fields in October). All samples were stored at -25 [degrees] C.
Chemicals
All solvents and chemicals used during extraction and quantification were at least of analytic-reagent grade. TbOH-17[alpha] and TbO were provided by Roussel-Uclaf (Paris, France), TbOH-17[beta] was purchased from Sigma (Deisenhofen, Germany), and MGA from ICN. Testosterone-[d.sub.3] and MGA-[d.sub.3] were provided by RIVM (Bilthoven, Netherlands).
Equipment
The HPLC system used for studies I, II, IV, and V included a pump module (model 420; Kontron Instruments, Neufahrn, Germany), an injector (model 210-A Valve; Beckman, Munchen, Germany), a column oven (Jetstream Plus; Beckman), a fraction collector (model Frac-100; Pharmacia, Uppsala, Sweden), and an RP-18 column (studies I, II and IV: LUNA, 250 mm x 4.6 mm, 5 [micro]m, Phenomenex, Aschaffenburg, Germany; study V: NUCLEOSIL EC 150/4.6, 100-5 C18, Macherey-Nagel, Duren, Germany).
For gas chromatography (study I) a GC-8000 apparatus (Fisons/Carlo-Erba, Altrincham, UK) and a DB-5 column, size 15 m x 0.25 mm, 0.25 [micro]m film thickness, (J&W Scientific, Folsom, CA, USA) were used with helium (5.0; Linde, Wiesbaden, Germany) as carrier gas.
We performed liquid chromatography (study III) using a pump module (model 2248; Pharmacia) with an injector (Rheodyne, Rohnert Park, CA, USA)and an RP-18 column (NUCLEOSIL CC 125/2, 120-5 C18; Macherey-Nagel).
We performed mass spectrometry (study I and III) on a Fisons/Micromass Platform II (Altrincham, UK).
For enzyme immunoassay analysis (study I, II, IV and V), we used a photometer (model Spectra Image) from Tecan (Crailsheim, Germany).
Quantification of TbOH in Liquid Manure and Solid Dung
We performed steroid extraction and purification using a method previously described for feces (18). The eluate of the solid-phase extraction was completely dried in vacuum, and the residue was resolved in 600 [micro]L 20% methanol.
We separated TbOH-17[alpha] from its metabolites TbOH-17[alpha] and TbO by HPLC on a C18 reverse-phase column. The injection volume was 500 [micro]L (of the purified extract) and the column was eluted at 25 [degrees] C with a mixture of methanol/water (65/35, v/v) at a flow rate of 1 mL/min. The fraction size was 330 [micro]L.
We quantified the hormone concentration in the HPLC fractions by enzyme immunoassay following the procedure described in literature (18,19). In liquid manure and solid dung samples before storage, we calibrated the assay for the main metabolite TbOH-17[alpha]. We calculated the concentrations of TbOH-17[beta] and TbO by their cross-reaction in relation to TbOH-17[alpha] (e.g., a measured concentration of 45 pg/g TbOH-17[beta] corresponded to an actual content of 31 pg/g; the cross-reaction of TbOH-17[beta] compared to TbOH-17[alpha] was 144%). In solid dung samples after storage, we quantified TbOH-17[alpha], TbOH-17[beta], and TbO using the corresponding specific calibration curves.
Quantification of TbOH in Soil
Because of the dilution effect when manure or dung is spread on the fields, only low concentrations of TbOH and its metabolites could be expected in soil, and analyte enrichment had to be performed. Therefore, 50 g of soil were suspended in 25 mL water and extracted twice with 15 mL tert-butyl methyl ether (TBME) (overnight at 20 [degrees] C, using an overhead shaker). The TBME phases of both extractions were combined and completely evaporated (60 [degrees] C, shaking water bath), and the residue was resolved in 0.5 mL 80% methanol. Purification and quantification proceeded as described above. We measured the concentrations of TbOH-17[alpha] and TbOH-17[beta] using the corresponding specific calibration curves, and we determined TbO by its cross-reaction in relation to TbOH-17[beta].
Validation of TbOH Determination
Validation was performed for the major metabolite TbOH-17[alpha]. We determined the limit of detection, which corresponds to the smallest measurable content of analyte, by analyzing negative samples and calculating the mean plus the 3-fold standard deviation of the resulting values. Accuracy and precision were determined as the recovery in fortified blank samples (carried out in triplicate) and the variation coefficient of these recovery experiments, respectively (Table 1).
The poor and varying recovery rates demanded internal standardization, but it was not possible to find standard substances that behaved proportionally to the analyte during extraction and purification. Therefore we had to perform external standardization by the mean recovery rates of 42, 32, or 30%, depending on the matrix.
Confirmation Analysis
To confirm the identity of TbOH residues, we analyzed two representative liquid manure samples with gas chromatography-mass spectrometry (GC-MS): one sample from the manure canal and one from the manure storage. Extraction and clean-up occurred as described above; we determined the heptafiuorobutyryl derivatives similarly to a method described elsewhere (20). From the manure sample from the canal we measured the following concentrations: 6.7 ng/g TbOH-17[alpha] and 0.20 ng/g TbOH-17[beta] with GC-MS compared to 4.1 ng/g TbOH-17[beta] and 0.18 ng/g TbOH-17[beta] with HPLC/enzyme immunoassay. In the manure sample from the storage tank, the agreement of the results was just as satisfactory: 3.1 ng/g TbOH-17[alpha] and 0.065 ng/g TbOH-17[beta] detected with GC-MS, compared to 1.6 ng/g TbOH-17[alpha] and 0.055 ng/g TbOH-17[beta] detected with HPLC/enzyme immunoassay. Interferences made us unable to determine the TbO contents with GC-MS.
Quantification of Melengestrol Acetate in Feces
We analyzed fecal samples from study III by liquid chromatography (LC)-MS to identify the parent compound MGA excreted in feces.
After addition of 5 ng internal standard (MGA-[d.sub.3]) per gram of sample, an aliquot of 4 g feces was suspended in 6 mL water and extracted twice with 10 mL petroleum ether (PE) under gentle rotation (at 40 [degrees] C overnight and for 1 hr, respectively). The residue of the combined PE phases was resolved in 1 mL acetonitrile/water (95/5, v/v) and defatted twice with 2 mL PE. After the acetonitrile phase was evaporated (in vacuum) and the residue was resolved in 0.5 mL 80% methanol, purification proceeded as described above. The eluate of the solid phase extraction was evaporated to dryness (in vacuum) and the residue resolved in 30 [micro]L acetonitrile. For LC-MS analysis we injected 20 [micro]L of the purified steroid extract under the following conditions: Acetonitrile/water/formic acid (50/50/1, v/v/v) served as mobile phase at a flow rate of 0.6 mL/min. Retention times were 6.72 min for MGA and 6.70 min for MGA-d3. The monitored ions after electrospray ionization were 397, 438, and 337 for MGA and 400, 441, and 340 for MGA-[d.sub.3]. We identified the substances by the corresponding retention time and the relative peak area of selected ions. For quantification we calculated the area of the base peak of MGA (m/z 397) and MGA-[d.sub.3] (m/z 400) and compared their ratio to a linear calibration curve, which we obtained by measuring a range of at least five standards.
Quantification of Melengestrol Acetate in Solid Dung
We determined MGA in solid dung analogously to its determination in feces, but with some modifications: We extracted 3 g of solid dung; after evaporation of the combined PE extracts we redissolved the residue in 0.5 mL 80% methanol and then defatted it twice; we eluted the solid-phase extraction columns with 1.5 mL 80% methanol; the eluate was evaporated to dryness and the residue resolved in 15 [micro]L acetonitrile; injection volume for LC-MS analysis was 10 [micro]L, and flow rate was 0.3 mL/min.
Validation of LC-MS Analysis
Accuracy, precision, and limit of detection followed the principles described for TbOH determination. The detection limit was 0.2 ng/g (signal to noise ratio 5:1). For validation parameters, see Table 1.
Quantification of MGA in Soil
Analysis of soil samples focused on MGA. Only small concentrations of MGA could be expected in soil, and analyte enrichment had to be performed. Because the sensitivity of LC-MS for the determination of MGA in soil was not sufficient, we had to apply enzyme immunoassay for quantification.
We extracted 50 g of soil with 30 mL methanol overnight and afterward centrifuged the sample. The supernatant was transferred to new extraction vials, diluted with water to a final concentration of 40% methanol, and extracted overnight with 15 mL PE. After the emulsion was centrifuged and frozen, the PE phase was decanted and evaporated to dryness in a shaking water bath (at 70 [degrees] C). The residue was resolved in 1 mL 80% methanol. After adding 2 mL water, we performed solid-phase extraction as described for solid dung samples. The eluate was evaporated to dryness (in vacuum) and resolved in 600 [micro]L 20% methanol. We separated MGA from interfering substances by HPLC on a C18 reverse-phase column. HPLC conditions (injection volume, mobile phase, flow rate, and column temperature) were the same as applied for analysis of TbOH samples; however, the fraction size was 250 [micro]L. The MGA content in the HPLC fractions was quantified by enzyme immunoassay (21) with a commercially available EIA-Kit (R-Biopharm, Darmstadt, Germany).
Validation of MGA Determination in Soil
The validation followed the principles described for TbOH determination. Table 1 shows the validation parameters. Similar to TbOH, internal standardization with structurally related steroids was not possible because all tested substances showed a different extraction effectiveness compared to MGA. For external standardization, all results were corrected by the mean recovery rate of 25%.
Results
TbOH
Residues in liquid manure. Figure 3 shows the residues of TbOH in liquid manure during collection in the manure canal (all values were corrected by the recovery rate). In the canal the manure was heterogeneous, and two samples collected at the same date represented different areas of the canal. As expected, TbOH-17[alpha] was the dominant metabolite, followed by TbOH-17[beta] and TbO. On average, the amount of TbOH-17[alpha] was 22 and 49 times as high, respectively, as the amount of TbOH-17[beta] and TbO.
[FIGURE 3 OMITTED]
Previous studies with implanted calves indicated that the mean plasma concentrations of TbA were relatively constant due to a constant release of TbA from the implant (22). This correlation probably explains our observation that the measured concentrations of the three detected metabolites reflected the number of treated animals in the stable. At the beginning of the collection, the manure canal contained manure from both treated and untreated animals because the collection reservoirs were emptied only every 2 weeks. On 23 September the maximum number of hormone preparations was applied to the animals. Afterward the animals were slaughtered consecutively, so that 13 fewer treated heifers contributed to the manure on 7 October.
The degradation of TbOH during 5.5 months of manure storage is illustrated in Figure 4 (all results were corrected by the recovery rate). The level of TbOH decreased from 1,700 pg/g in the beginning to 1,100 pg/g (17[alpha]-isomer) and from 160 pg/g to 100 pg/g (17[beta]-isomer). These values corresponded to a half-life of 267 and 257 days, respectively, whereas for TbO we observed no decline, possibly because of oxidation of TbOH-17[alpha] and -17[beta].
[FIGURE 4 OMITTED]
The half-lives of TbOH-17[alpha] and TbOH-17[beta] were calculated according to the following formula, usually applied for radioactive decay kinetics:
c(t) = c(0) x [e.sup.-[lambda]t],
where t is time; c(t) is concentration at time t; c(0) is concentration at the beginning; and [lambda] is the constant of decay. Thus, the half-life is given by
[t.sub.1/2] = -ln (1/2)/[lambda].
Contents in Solid Dung. As in liquid manure, TbOH-17[alpha] was the main metabolite of TbOH in solid dung. However, in 4 of 10 analyzed samples the amount of TbO exceeded that of TbOH-17[beta] (Table 2). Compared to liquid manure the TbOH contents in solid dung before storage were 5-70 times higher, depending on the position in the dung hill. TbOH was eluted with rainwater passing the dung hill and gathering at the effluent. Although TbOH was partly degraded during 4.5 months of storage, it could be detected in four of six samples (levels up to 10 ng/g TbOH-17[alpha], 0.3 ng/g TbOH-17[beta], and 0.8 ng/g TbO).
The huge variation of the measured concentrations reflected the heterogeneity of the dung hill caused by erection and transportation procedures.
Residues in Soil TbOH-17[alpha], TbOH-17[beta], and TbO could be detected in soil fertilized with liquid manure and solid dung. The dilution effect when manure and dung were spread on the fields made the maximum concentrations in soil markedly lower (Table 3).
The first soil samples were taken 31 days after fertilization with fresh liquid manure in autumn. Assay of these samples indicated that TbOH residues originating from liquid manure were stable for less than a month. We confirmed this result by analyzing the soil samples fertilized in spring with stored manure. TbOH was traceable 8 days after spreading on the fields, but could not be quantified after 40 days.
TbOH concentrations in soil fertilized with stored solid dung were lower than in soil fertilized with liquid manure. However, residues were detectable 58 days after fertilization. This potential greater stability might be caused by adsorption of TbOH to straw material, which possibly protected the substances from degradation or leaching.
MGA
Residues in feces. The data in Table 4 demonstrate that MGA residues in feces were clearly dose dependent. Average levels during 1-, 3-, and 10-fold treatment were 2.1, 5.9, and 16.2 ng/g, respectively. The concentrations 24 hr after feeding were approximately 1.4 times higher than after 12 hr, reflecting the passage through the digestive tract.
Contents in solid dung. The MGA amounts in solid dung before storage ranged between 260 and 7,760 pg/g. After 4.5 months of storage the concentrations still ranged between 420 and 6,030 pg/g (Table 5). In comparison with TbOH the decrease was not so clear, owing to a greater stability of MGA. But like TbOH, the varying MGA concentrations reflected the heterogeneity of the dung hill caused by erection and transportation conditions.
Residues in Soil. In soil samples MGA originating from solid dung was traceable from spring until the end of the cultivation period in October (Table 3). The experimental fields were thoroughly ploughed 195 days after fertilization; thus continuation of sampling seemed inappropriate. As described for TbOH, the maximum MGA concentrations in soil were definitely lower than for solid dung because of the dilution effect when dung was spread on the fields.
Discussion
After the use of TbA and MGA as growth promoters, we analyzed the degradation of their residues in solid dung and liquid manure. In soil fertilized with solid dung, TbOH and MGA were traceable for 58 and 195 days, respectively.
Studies I and II
After excretion via feces, TbOH could be detected in liquid manure and solid dung in significant concentrations. With the help of a simplified model calculation illustrated in Table 6, we tried to estimate the recovered fraction of the total applied dose. The determined values between 3 and 42% are significant, considering the fact that in the United States, for example, presumably several tons of TbA are applied every year. In some circumstances, discussed below, the total concentration of TbOH metabolites was probably even higher.
In cows the biliary excretion of TbOH predominates. Ten metabolites with 3-oxotriene-structure and three additional compounds that had lost their 3-oxotriene-structure could be identified in cow bile (9). However, our quantification system is validated and suitable only for the metabolites TbOH-17[alpha], TbOH-17[beta], and TbO.
TbOH is known for its ability to bind to biologic macromolecules, especially proteins. Studies with radiolabeled TbA implants in heifers proved that about 90% of the total radioactivity could not be extracted with commonly used organic solvents and either was water-soluble or an insoluble tissue-bound residue (24). If TbOH residues are also bound to fecal compounds, the extraction and measuring methods we applied in our studies underestimated the actual concentrations in liquid manure and solid dung.
Studies on the stability of TbOH in bovine urine showed that storage of urine samples in direct sunlight led to decreased TbOH concentrations (25). Storage of feces samples at room temperature in some cases caused partial or complete loss of the TbOH-17[alpha] content (26). Throughout our experiments, dung hills, the manure collection canal, and storage pit were neither cooled nor protected from sunlight.
Because other steroid hormones (e.g., estrone) can be catabolized by microorganisms (27), microbial degradation of TbOH is conceivable as well. However, knowledge of microbial metabolism of steroids is still scarce. In an in vitro study performed with Escherichia coli and Clostridium perfringens as representative intestinal microorganisms, no specific effect on the concentration of the hormone 4-pregnene-20[beta]-ol-3-one was observed (28).
Finally, the actual amount of metabolites may have been higher because small amounts of TbOH were eluted with rainwater passing over the dung hill, and the adsorption of TbOH to straw material cannot be excluded.
Previous studies performed by Haase et al. (29) and Rumsey et al. (30) demonstrated that synthetic hormones (namely, diethylstilbestrol) were stable in liquid manure stored under anaerobic conditions. Similarly, degradation of TbOH occurred rather slowly. Its half-life in liquid manure without ventilation was approximately 260 days.
In a dung hill erected with excrement from pregnant heifers and stored for several months, estrogen concentrations up to 780 ng/g were measured (31). In this study, however, more degradation was observed in the anaerobic lower layers of the dung hill.
Study III
As for TbOH, we attempted to evaluate the recovered fraction of the total applied dose of MGA using a simplified model (Table 7). The calculated excretion rate via feces (12%) confirmed preceding observations that about 15% of the daily administered dose passed through the gastrointestinal tract unabsorbed. Bile cannulation studies showed that the primary route of excretion from the body was via the bile. However, the metabolic fate of MGA in heifers has not been investigated in detail until now. Although MGA was primarily excreted unmodified, several metabolites were found in the non-MGA fraction in liver (11). At least three of them are hormonally active substances; they exhibited binding affinities to the bovine uterine progestin receptor (bPR) between 28 and 85% in comparison to progesterone (10). Because our measurement method was specific only for the parent compound, our results cannot give a complete picture concerning the actual total residues. Microbial degradation and adsorption to straw might also have contributed to a reduced recovery of the parent substance in relation to the total applied dose.
Studies IV and V
TbOH and MGA originating from contaminated excrement were detectable in soil up to several months after fertilization. From our field experiments we cannot deduce the mechanisms of how these hormones disappear from soil, but it is known that steroids can interact with humic substances and form stable products (32). By comparing to the behavior of other well-known agricultural or industrial soil pollutants, we can speculate on the fate of TbOH and MGA. They can be degraded by soil bacteria and/or photochemical reactions (UV light). Rain might wash the substances into lower soil horizons or directly into surface water without passing the soil column. Both processes might be promoted by dissolved organic matter that can bind the steroids and enhance their solubility and mobility in the aqueous phase. Strong adsorption of hydrophobic compounds to soil particles is well documented for many agricultural and industrial chemicals. Thus, persistent organic chemicals accumulate, whereas weaker adsorption might result in transposition to ground and surface water. Big hydrophobic molecules are generally more strongly adsorbed than small hydrophilic molecules (33,34).
In conclusion, research on the stability and degradation of sex hormones should be a crucial element of an environmental risk evaluation.
REFERENCES AND NOTES
(1.) Shore LS, Shemesh M, Cohen R. The role of oestradiol and oestrone in chicken manure silage in hyperoestrogenism in cattle. Aust Vet J 65:68 (1988).
(2.) Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken R-D, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants. I. Investigations in Germany, Canada and Brazil. Sci Total Environ 225:81-90 (1999).
(3.) Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chem Ecol 8:275-285 (1994).
(4.) Tabak HH, Bloomhuff RN, Bunch RL. Steroid hormones as water pollutants. II. Studies on the persistence and stability of natural urinary and synthetic ovulation-inhibiting hormones in untreated and treated wastewaters. Dev Ind Microbiol 22:497-519 (1981).
(5.) Tabak HH, Bunch RL. Steroid hormones as water pollutants. Dev Ind Microbiol 11:367-376 (1970).
(6.) Neumann F. Pharmacological and Endocrinological Studies on Anabolic Agents. In: Anabolic Agents in Animal Production (Lu FC, Rendel J, eds). Stuttgart:Georg Thieme Publishers, 1976;253-264.
(7.) Meyer HHD, Rapp M. Reversible binding of the anabolic steroid trenbolone to steroid receptors. Acta Endocrinol 108(suppl 267):t29 (1965).
(8.) Danhaive PA, Rousseau GG. Binding of glucocorticoid antagonists to androgen and glucocorticoid hormone receptors in rat skeletal muscle. J Steroid Biochem 24:481-487 (1986).
(9.) Pottier J, Cousty C. Differences in the biotransformation of a 17[alpha]-hydroxylated steroid, trenbolone acetate, in rat and cow. Xenobiotica 11:489-500 (1981).
(10.) Bauer ERS, Daxenberger A, Petri T, Sauerwein H, Meyer HHD. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and the bovine gestagen receptor. APMIS 108:838-846 (2000).
(11.) Lauderdale JW, Goyings LS, Krzeminski LF, Zimbelman RG. Studies of a progestogen (MGA) as related to residues and human consumption. J Toxicol Environ Health 3:5-33 (1977).
(12.) Patterson DJ, Kiracofe GH, Stevenson JS, Corah LR. Control of the bovine estrous cycle with melengestrol acetate (MGA): a review. J Anim Sci 67:1895-1906 (1989).
(13.) Lauderdale JW. Use of MGA[R] (melengestrol acetate) in animal production. In: Proceedings of the Symposium on Anabolics in Animal Production: Public Health Aspects, Analytical Methods, and Regulation (Meissonnier E, Mitchell-Vigneron J, eds), 15-17 February 1983, Paris, France. Paris:Office International des Epizooties, 1983;193-212.
(14.) Duncan GW, Lyster SC, Hendrix JW, Clark JJ, Webster HD. Biological effects of melengestrol acetate. Fertil Steril 15:419-432 (1964).
(15.) Hageleit M, Daxenberger A, Kraetzl W-D, Kettler A, Meyer HHD. Dose-dependent effects of melengestrol acetate (MGA) on plasma levels of estradiol, progesterone and luteinizing hormone in cycling heifers and influences on oestrogen residues in edible tissues. APMIS 108:847-854 (2000).
(16.) Food and Agriculture Organization of the United Nations. Hormones in animal production. FAO Animal Production and Health Paper 31. Rome:United Nations Food and Agriculture Organization, 1982.
(17.) Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. Washington, DC:National Academy Press, 1980.
(18.) Meyer HHD, Hoffmann S. Development of a sensitive microtitration plate enzyme-immunoassay for the anabolic steroid trenbolone. Food Addit Contam 4:149-160 (1987).
(19.) Meyer HHD. Enzymimmunologische Me[beta]verfahren zur Hormonanalytik. Stuttgart:Ferdinand Enke Verlag, 1989.
(20.) Daeseleire E, de Guesdquiere A, van Peteghem C. Derivatisation and gas chromatographic-mass spectrometric detection of anabolic steroid residues isolated from edible muscle tissues. J Chromatogr 562:673-679 (1991).
(21.) Hageleit M, Daxenberger A, Meyer HHD. A sensitive enzyme immunoassay (EIA) for the determination of melengestrol acetate (MGA) in adipose and muscle tissues. Food Addit Contain 18:285-291 (2001).
(22.) Evrad P, Maghuin-Rogister G, Rico AG. Fate and residues of trenbolone acetate in edible tissues from sheep and calves implanted with tritium-labeled trenbolone acetate. J Anita Sci 67:1489-1496 (1989).
(23.) Daxenberger A, Lange IG, Meyer K, Meyer HHO. Detection of anabolic residues in misplaced implantation sites in cattle. J Assoc Off Anal Chem 83:809-819 (2000).
(24.) Ryan JJ, Hoffmann B. Trenbolone acetate: experience with bound residues in cattle tissues. J Assoc Off Anal Chem 61:1274-1279 (1978).
(25.) van der Merwe PJ, Pieterse JW. Stability of zeranol, nandrolone and trenbolone in bovine urine. Analyst 119:2651-2653 (1994).
(26.) Vogt K. Radioimmunologische Bestimmung von Trenbolon in Urin, Galle und Kot von Mastkalbern nach subkutaner Implantation von Revalor[R]. Arch Lebensmittelhyg 35:27-32 (1984).
(27.) Groh H, Schade K, Horhold-Schubert C. Steroid metabolism with intestinal microorganisms. J Basic Microbiol 33:59-72 (1993).
(28.) Schlenker G, Muller W, Glatzel P, Birkelbach C. Experimentelle Untersuchungen zum Einflu[beta] von Escherichia coli und Clostridium perfringens auf das Steroid 4-Pregnen-20[beta]-ol-3-on. Berl Munch Tierarztl Wschr 111:418-421 (1998).
(29.) Haase E, Agthe O, Megnet R. Uber den Abbau von Diethylstilbestrol (DES) in Kalbergulle. Dtsch tierarztl Wschr 89:477-479 (1982).
(30.) Rumsey TS, Miller RW, Dinius DA. Residue content of beef feedlot manure after feeding diethylstilbestrol, chlortetracycline and ronnel and the use of stirofos to reduce population of fly larvae in feedlot manure. Arch Environ Contam Toxicol 6:203-212 (1977).
(31.) Mostl E, Dobretsberger A, Palme R. Ostrogenkonzentration im Stallmist trachtiger Rinder. Wien Tierarztl Mschr 84:140-143 (1997).
(32.) Ziechmann W, Muller-Wegener U. Bodenchemie. Mannheim:BI-Wissenschaftsverlag, 1990.
(33.) Scheffer F, Schachtschnabel P. Lehrbuch der Bodenkunde. Stuttgart:Ferdinand Enke Verlag, 1998.
(34.) Blume H-P. Handbuch des Bodenschutzes. Landsberg/ Lech:ecomed verlagsgesellschaft, 1992.
Bettina Schiffer, (1) Andreas Daxenberger, (1) Karsten Meyer, (2) and Heinrich H.D. Meyer (1)
(1) Institute of Physiology and (2) Institute of Animal Hygiene, TU Munchen-Weihenstephan, Freising-Weihenstephan, Germany
Address correspondence to B. Schiffer, Institute of Physiology, TU Munchen-Weihenstephan, Weihenstephaner Berg 3, D-85354 Freising-Weihenstephan, Germany. Telephone: + 49 8161 715546. Fax: + 49 8161 714204. E-mail: schiffer@ weihenstephan.de
These studies were financially supported by the European Union.
Received 28 December 2000; accepted 19 April 2001.
COPYRIGHT 2001 National Institute of Environmental Health Sciences
COPYRIGHT 2004 Gale Group