ABSTRACT
The phototoxicity of triamcinolone 16,17-acetonide has been estimated through a panel of in vitro tests. The main target involved in phototoxicity induced by triamcinolone appeared to be the cell membrane. Oxygen-independent photohemolysis was observed. A photochemical study in water and buffered solutions supported the conclusion that this is related to the action of radicals formed upon UV irradiation (in particular UV-B) by Norrish Type-I fragmentation of the C-20 ketone group. Peroxy radicals were formed in the presence of oxygen and were the active species in that case. Three photoproducts, isolated from the photodegradation of the drug, were submitted to the same toxicity tests. Two of them were proved to possess toxic or phototoxic properties on erythrocytes, primarily induced by UV-B light, and may participate in the photosensitizing activity of triamcinolone 16,17-acetonide. Our in vitro results suggest that the drug can elicit weak photosensitizing properties in vivo.
Abbreviations: BHA, 2,6-di-tert-butylhydroxyanisole; BPB, bromophenol blue; BSA, bovine serum albumin; DMEM, Dulbecco modified Eagle medium; DMTU, N,N'-dimethylthiourea; EDTA, ethylenediaminetetra-acetic acid; HPLC, high-performance liquid chromatography; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NaN^sub 3^, sodium azide; NBT, Nitro blue tetrazolium; PBS, phosphate-buffered saline; RBC, red blood cells; RNO, p-nitrosodimethylaniline; SDS, sodium dodecylsulphate; SOD, superoxide dismutase.
© 2003 American Society for Photobiology 0031-8655/03 $5.00+0.00
INTRODUCTION
Glucocorticosteroids, natural hormones derived from [alpha]-pregnane, are potent therapeutic agents for the treatment of a broad range of inflammatory diseases. Semisynthetic derivatives are widely used systemically, mainly for the treatment of rheumatoid diseases and allergic manifestations such as severe asthma (1), and many of them are effective as topical application in dermatoses and other dermatological diseases.
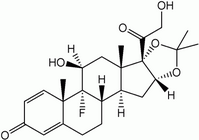
Triamcinolone (9-fluoro-11[beta], 16[alpha], 17,21-tetrahydroxypregna-1,4-diene-3,20-dione) was introduced in 1958 as a representative of halogenated [Delta]-corticosteroids, a novel class bearing on the steroid skeleton both a fluorine atom in position 9 and a double bond between positions 1 and 2 on Ring A. It is already known that the change in the geometry of Ring A, induced by the introduction of an additional double bond in positions 1-2 ([Delta]^sup 1^-corticoids), caused higher activity with respect to the parent compounds (such as cortisone and hydrocortisone).
Furthermore, the presence of 9[alpha]-fluorine not only enhances the anti-inflammatory potency but also markedly increases the mineralo-corticoid activity, which is an undesirable effect, i.e. during systemic administration for rheumatoid arthritis. However, the introduction of the 16[alpha]-hydroxy group can attenuate the mineralo-corticoid activity. Intramuscular and topical administration of triamcinolone, generally dispensed as the more potent acetonide derivative (16[alpha], 17[alpha]-cyclic ketal or isopropylidene derivative), are successfully used for the treatment of dermatoses (2,3) and, in particular, of psoriasis (4,5), one of the few inflammatory dermatoses that does not respond to largely used topical steroids. Furthermore, this corticosteroid is used against drug-induced erythema (6) and for the topical chemotherapy of pigment abnormalities in surgical patients (7). However, in this last application a moderate photoallergy has been noted, and, furthermore, it is known that this drug photobinds to the human mineralocorticoid receptor (8). In view of these applications, it is obviously important to ascertain that this drug does not itself cause an adverse photoeffect.
On the other hand, it is well known that triamcinolone is highly photoreactive both in the solid state (9,10) and in organic solvents (11). It has been demonstrated that the course of the photochemical reaction is wavelength dependent, with a different product distribution under UV-A and UV-B irradiation.
These facts motivated the present study, which compares photobiological and photochemical data. The previous photochemical study in organic solvents has been extended to diluted aqueous solution (buffered, oxygen or argon equilibrated), and the in vitro effects on murine erythrocytes have been determined using photohemolysis as an endpoint. Furthermore, the cellular phototoxicity on murine fibroblasts and the photochemical damage induced by triamcinolone on biological molecules such as lipids, proteins and DNA have been explored to better characterize the cellular targets involved in the phototoxic reactions. Because, as mentioned above, the drug is quite photoreactive, the photodegradation products have been isolated, and in vitro tests on their phototoxic effects have been carried out.
MATERIALS AND METHODS
Chemicals
Triamcinolone acetonide was supplied by Farmabios, Gropello Cairoli, Italy. The purity of the material was checked using high-performance liquid chromatography (HPLC) analysis and nuclear magnetic resonance spectroscopy. N,N-Dimethyl-4-nitrosoaniline (RNO) and imidazole were obtained from Merck Schuchardt (Darmstadt, Germany). Nitro blue tetrazolium (NBT), linoleic acid, bovine serum albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), sodium azide (NaN^sub 3^), N,N'-dimethylthiourea (DMTU), superoxide dismutase (SOD), 2,6-di-tert-butylhydroxyanisole (BHA) and agarose were obtained from Sigma Chemical Company (St. Louis, MO). pBR322 DNA, ethidium bromide solution, acrylamide, N,N'-methylene-bis-acrylamide, ammonium persulphate and N,N,N',N'-tetramethylethylenediamine were purchased from Pharmacia Biotech AB (Uppsala, Sweden). Coomassie brilliant blue R-250 was purchased from Bio-Rad Laboratories (Segrate, Italy). Spectroscopic grade solvents were used.
Photochemical reactions
Triamcinolone solutions (1 to 2 × 10^sup -5^ M) in water, buffered solution, or organic solvents in serum-capped quartz tubes were purged with argon or oxygen as appropriate and externally irradiated in a multilamp apparatus fitted with two 15 W phosphor-coated bands (Helios Italquartz, Milan, Italy; center of emission 310 or 350 nm, respectively, band width ca 40 nm).
HPLC analysis. The course of the reaction was monitored by HPLC analysis (see below) on the basis of appropriate calibration curves, both for the starting material 1 and for photoproducts 2-4, the isolation and characterization of which have been previously reported (12).
In a typical experiment triamcinolone (1 × 10^sup -5^ M in phosphate-buffered saline [PBS]) was irradiated as above. After irradiation all samples were filtered (0.22 µm cellulose acetate filter membranes, Waters Italia, Vimodrone, Italy) before HPLC elution. Analytical HPLC analysis was performed by a Varian ProStar HPLC (Walnut Creek, CA), equipped with two Pro Star 210 solvent delivery modules and a Pro Star320 UV/Vis Detector, using a reverse phase column C-18 (Waters Spherisorb, S5 ODS2, 4.6 × 250 mm, 5 'm). The mobile phase consisted of a mixture of acetonitrile-water (60:40, vol/vol) with a flow rate of 0.5 mL/min. The eluted species were detected at both 238 and 276 nm.
Production of reactive oxygen species. For singlet oxygen determination, samples containing 1 (2.2 × 10^sup -5^ M), RNO (4 × 10^sup -5^ M) and imidazole (4 × 10^sup -5^ M) in phosphate buffer (0.02 M, pH = 7.3) were irradiated with increasing UV-B and UV-A doses (0-30 J/cm^sup -2^), and their absorbance at 440 nm was then measured (13).
For superoxide anion generation, samples containing 1 (1 × 10^sup -5^ M) and NBT (1.6× 10^sup -4^ M) in carbonate buffer (pH = 10) were irradiated for increasing times, and their absorbance at 560 nm was measured (14).
Phototoxicity tests
Irradiation procedure. Two Philips HPW 125 lamps, emitting mainly at 366 nm, or one Philips PL-S 9W/12 lamp, emitting mainly at 312 nm, were used for irradiation experiments. The total energy was detected by a radiometer (Mod. 97503, Cole-Parmer Instrument Company, Niles, IL) equipped with a 365-CX or 312-CX sensor. Increasing UV-B and UV-A doses were used, under controlled room temperature, up to 5 and 25 J/cm^sup 2^, respectively. The samples were maintained at room temperature during irradiation.
Photohemolysis. Whole blood, collected from untreated albino mouse using heparin as anticoagulant, was washed with PBS (0.01 M phosphate buffer, 0.135 M NaCl, pH 7.4) and centrifuged (2500 rpm for 15 min), and the supernatant and the buffy coat were discarded. The procedure was repeated until the supernatant was colorless. Red blood cells (RBC) (10^sup 6^ cells/mL, corresponding to OD^sup 650^ = 0.6-0.7) were resuspended in PBS and used within 48 h. For the photohemolysis experiments, small volumes (less than 1%) of concentrated ethanol solutions of the compounds were added to RBC (final concentration 50 'M) and incubated for 15 min at 37°C in the dark. The suspension was then irradiated with increasing UV-B and UV-A doses under gentle shaking in a controlled temperature bath. Scavengers, dissolved in PBS and then mixed with RBC, were used at the following concentrations: BHA, 40 'M; NaN^sub 3^, 150 'M; SOD, 1.5 'M; DMTU, 5 mM.
Hemolysis was followed by monitoring of the decrease in light scattering at 650 nm due to intact cells (15). Control samples were (1) untreated RBC (0% hemolysis); (2) RBC diluted 1:1000 with water (100% hemolysis); (3) RBC in the presence of compounds and kept in the dark; and (4) RBC irradiated without compounds.
Linoleic acid peroxidalion. Linoleic acid (1 × 10^sup -3^ M) in PBS (0.01 M phosphate buffer, pH 7.4, 0.135 M NaCl) containing 0.05% Tween 20 as emulsifying agent was irradiated in the presence of triamcinolone (1 × 10^sup -5^ M) with increasing UV-A and UV-B doses as described by Zhou and Moore (16). The peroxidation of linoleic acid was monitored at 233 nm by the absorbance of the peak corresponding to the conjugated dienic hydroperoxides formed during irradiation.
Protein photodamage. For the determination of protein photocross linking, ghosts were prepared by following the gradual osmotic lysis method (17). White membranes (ghosts) were resuspended in PBS. Membrane protein contents were determined as described by Peterson (18), using BSA as a standard. The compounds were added (100 µM) to the membrane suspension (3.0 mg/mL protein concentration) and incubated in the dark for 15 min before UV-B and UV-A irradiation. Samples (300 µL) in 1 mm quartz cuvettes were irradiated (23.6 J/cm^sup 2^) in a controlled temperature water bath (25°C). The membrane samples (100 µg) were reduced and denatured by addition of [beta]-mercaptoethanol and sodium dodecylsulphate (SDS) at 90°C for 3 min, and bromophenol blue (BPB) was added before polyacrylamide gel electrophoretic analysis (8% running gel, 4% stacking gel). The electrophoresis was carried out at 100 V for 4 h in a Bio-Rad Power Pac 300 apparatus, using 0.124 M Tris, 1 mM glycine and 0.5% SDS as running buffer. The gel, stained with Coomassie brilliant blue R-250 (2.5 mg/L) dissolved in methanol-acetic acid-water (45.4:4.6:50, vol/vol/vol), was fixed, washed with a methanol-acetic acid-water mixture (5:7:88, vol/vol/vol) and submitted to densitometric analysis by the image analyzer software Quantity One (Bio-Rad). Controls were (1) untreated ghosts; (2) ghosts irradiated without the compounds; and (3) ghosts with the compounds but kept in the dark.
Photocytotoxicity. Balb/c mouse 3T3 fibroblasts were grown in Dulbecco modified Eagle medium (DMEM; Sigma-Aldrich, Milano, Italy) supplemented with 115 units/mL of penicillin G, 115 g/mL of streptomycin and 10% fetal calf serum (all from Life Technologies, Milano, Italy). Individual wells of a 96-well tissue culture microtiter plate (IWAKI Glass Co, Tokyo, Japan) were inoculated with 100 µL of DMEM containing 5 × 10^sup 3^ 3T3 cells. The plate was incubated at 37°C in a humidified 5% CO2 incubator for 72 h to form a monolayer of approximately 100% confluence.
The medium was removed, and 100 µL of the compounds dissolved in ethanol and diluted with Hank balanced salt solution (pH = 7.2) was added to each well. Final concentrations were 25, 50 and 100 µM, ethanol never exceeding 1%. The plate was then incubated for 15 min, and the control plate was placed in the dark. After irradiation, the solution was replaced by the medium, and the plates were stored in the incubator for 24 h. Cell viability was assayed by the MTT test as already described (19).
DNA photodamage. For determination of DNA strand breaks, each pBR322 DNA sample (100 ng) was dissolved in Tris-ethylenediaminetetra-acetic acid (EDTA) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) and irradiated with increasing UV-B and UV-A doses in the presence of the compounds under examination (50 µM). The samples were loaded on 1% agarose gel (7.6 × 10 × 0.4 cm) after addition of 1 µL of gel-loading buffer (0.25% BPB, 0.25% xylene cyanol and 40% wt/vol sucrose) to each of them. The electrophoretic run was carried out in Tris-acetate-EDTA buffer (0.04 M Tris-acetate, 1 mM EDTA) at 100 V for 2 h, using a GNA-100 electrophoretic apparatus (Pharmacia).
After staining in ethidium bromide solution (1 µg/mL in the same buffer) for 20 min, the gel was washed with water, and the DNA bands were detected under UV radiation with a UV transilluminator. Photographs were taken with a digital photocamera Kodak DC256, and the quantitation of the bands was achieved by the image analyzer software Quantity One (Bio-Rad). The fraction of supercoiled-DNA was calculated as described (20).
RESULTS AND DISCUSSION
Photochemistry of triamcinolone
The photoreactions of triamcinolone 1 had been examined previously in relatively concentrated (2 to 5 × 10^sup -3^ M) solutions in some organic media (12). Because the photobiological experiments were necessarily carried out in (buffered) aqueous solutions, the photochemistry of this drug was now explored under such conditions. This imposed a limit on the concentration (2 × 10 M). At this concentration the photochemistry was studied in aqueous medium as well as in some organic solvents, both in argon-purged and in oxygen-saturated solutions. The irradiation was carried out using broadband emission lamps, centered at 310 and 350 nm, with a half-intensity width of ca 40 nm. These were representative of UV-B and UV-A, respectively. As it appears from Fig. 1, the spectrum of compound 1 shows only a weak tail in both these regions.
As calculated by HPLC analysis, irradiation by UV-B at this concentration led to the consumption of ca 50% of compound 1 within 6 and 12 min (corresponding to 0.5 and 1 J/cm^sup 2^, respectively), the longer time applying to MeCN and the shorter one to iso-propanol and aqueous media. In all cases, oxygen-saturated solutions reacted about two-thirds as fast as argon-flushed solution.
The products obtained are indicated in Scheme 1, and the product distribution at ca 50% conversion is reported in Table 1. The major photoproduct formed in argon-purged organic solvents was dienone 3, which was accompanied by a minor amount of a product isomeric with the starting material, ketone 2, resulting from the well-known "lumiketone" rearrangement of the cross-conjugated dienone moiety in Ring A (21,22). These were the same products obtained at a higher concentration. On the contrary, in water or in phosphate buffer the only product detected was the rearranged ketone 2 formed in a yield (10%) close to that observed in organic solvent, with no appreciable amount of product 3. Adding 10% of methanol to the buffer (other organic solvents such as ethanol or isopropanol gave similar results) caused the formation of 3 again, although in a lower yield than in pure organic solvents, whereas the formation of 2 was unaffected.
In oxygen-saturated solutions the yield of ketone 2 remained unchanged, but compound 3 was not observed, and it was substituted by a different product, hydroperoxide 4. Only in buffer containing 10% MeOH was some of the diketone 3 formed, along with products 2 and 4.
As for the irradiation with UV-A, this gave rearranged ketone 2 as virtually the only product. The results were the same in argon- and in oxygen-saturated solutions. The yield was satisfactory in organic solvent (50% in acetonitrile) and poor in (buffered) aqueous solution (5-7%).
These results can be rationalized on the basis of the previous mechanistic investigation. Two separate chromophores are present in compound 1, and the primary photochemical step depends on selective excitation of either one. Irradiation at 350 nm (as well as at 254 nm, not considered here because it is not relevant in the environment) involves exclusive excitation of the cross-conjugated ketone in Ring A. This undergoes the "lumi-rearrangement," typical of such chromophores (path a in Scheme 1) and gives product 2 (50% in MeCN). This remains the only photoprocess in aqueous media, where the low yield of 2 is due to fast secondary pholodecomposition of this product (which absorbs more strongly than 1 or 2 in this region, see Fig. 1), as known in the literature for related compounds (23) and checked in preliminary experiments.
At 310 nm the isolated ketone at C^sub 20^ absorbs ca 50% of the light, and a different path becomes available, homolytic (Norrish Type I) cleavage, to give alkyl radical 5 (path b in the Scheme). Thus, the yield of 2 is much reduced, and the main product is monoketone 3, arising from 5 by hydrogen atom abstraction from the reaction medium. A (buffered) aqueous medium is not a hydrogen atom donor, and in this case the radical decays to unidentified products, presumably highly polar compounds resulting from degradation of Ring D. Adding a hydrogen donor (see the experiments in PBS-MeOH) restores the formation of 3 to some degree. As one may expect, radical 5 is trapped by oxygen, and this leads to the formation of peroxy radical 6 and hydroperoxide 4. The latter compound is formed both in organic and in aqueous media (except in MeCN, apparently not a sufficient H donor for converting 6 to 4; we showed previously that 4 is also formed in this solvent in the presence of thiols, however. Formation of some hydroperoxide 4 in water may involve previous reduction of the relatively stable peroxyl radical). Summing up, the primary photoprocesses of compound 1 in aqueous media are the same as those in organic solvents, although the fate of the thus formed intermediates is affected to some degree by the medium. Quantum yields are difficult to determine with exactness, because of the quite low absorption, but because the rates of decomposition in diluted (10-5 M) aqueous and organic media are within a factor of 2, it is reasonable to expect that [Phi] is close to the values determined in more concentrated (10^sup -3^ M) organic solvents, viz. 0.3-0.5 (12).
The efficiency of triamcinolone in generating singlet oxygen and Superoxide anion was tested. The former was evaluated by measuring the bleaching at 440 nm of RNO induced by imidazole as a singlet oxygen-specific substrate (24) and the latter by the reduction of NBT to formazan (14). Both assays revealed that triamcinolone under either UV-B or UV-A light generates a small amount of reactive oxygen species (data not shown), the yields being more than 10 times lower than those produced by other photosensitizers, such as quinolone antibiotics or 8-methoxypsoralen, that we compared under the same conditions (19,25). This could be in part due to the lower absorption with respect to those substrates in this wavelength range.
In vitro phototoxicity assays with triamcinolone and its photoproducts
Photohemolysis was evaluated by irradiating murine balb/C mouse RBC (10^sup 6^ cells/mL) with UV-A or UV-B light in the presence of triamcinolone (1, 50 µm). Neither triamcinolone in the dark nor light alone was able to induce hemolysis at the doses tested.
As shown in Fig. 2, the photohemolytic activity of triamcinolone was elicited by much lower doses of UV-B (panel A) than UV-A light (panel B) because the total cell disruption was reached under about 1 J/cm^sup 2^ of UV-B and 20 J/cm^sup 2^ of UV-A. These light doses roughly correspond to the absorptivity of tramcinolone in those regions. The assay was repeated under various conditions to get deeper mechanistic evidence. Thus, hemolysis was slightly more efficient in the absence of oxygen with UV-B and markedly so with UV-A (Fig. 3). Four antioxidants were tested, viz. DMTU, BHA, SOD and NaN^sub 3^. The slow UV-A hemolysis was retarded to some extent both by BHA and NaN^sub 3^, whereas with UV-B, hemolysis was strongly retarded by BHA, the other additives having little effect.
The larger effect of UV-B is in part due to the better absorption of 1 in this wavelength range. However, the strong protection by BHA, a radical scavenger, and the fact that the protective effect is more apparent with UV-B clearly support a radicalic mechanism. As seen above, radical fragmentation (path b in Scheme 1) is the major path in this wavelength range. Alkyl radical 5 appears to be the active species in the observed membrane damage, which is suppressed by BHA. Oxygen quenches triplet 1 but then traps radical 5 to form peroxy radical 6, an electrophylic intermediate that is even more damaging than 5; hence, oxygen only slightly attenuates the damage with UV-B. On the contrary, concerted rearrangement to 2 (path a) is the main process with UV-A and does not involve aggressive intermediates that may damage the membrane, the small effect observed probably being related to the minor component at short wavelength of this source, which activates path b to some degree (see the moderate effect of BHA). Moreover, path a results in the formation of photoproduct 2, which is able to induce phototoxicity. In this case, triplet quenchers such as oxygen and NaN^sub 3^ exert a stronger protecting effect. NaN^sub 3^ is also a singlet oxygen quencher (24), but because singlet oxygen generation is minimal under these conditions (see above), ^sup 1^O^sub 2^ is not involved, and the same holds for Superoxide anion, as shown by the negative evidence with SOD.
The role of the photoproducts 2-4 in the photohemolysis also has been studied. Figure 4 shows the effect of the three products on RBC by simple incubation in the dark (panel A) or by further irradiation with UV-B (panel B) or UV-A (panel C). Compound 4 is able to totally hemolyze RBC in the dark (indeed, hydroperoxides undergo slow thermal hemolysis and can themselves be considered reactive oxygen species). Dark hemolysis is complete after less than half an hour of incubation. Furthermore, this compound is also strongly photoactive: hemolysis is complete upon administration of 0.4 and 4 J/cm^sup 2^ of UV-B and UV-A light, respectively. The light doses are also smaller than those required for obtaining the same effect with the parent drug. Indeed, hydroperoxides are known to undergo easy photosensitized homolysis through energy or electron transfer (26-28). Therefore, this hydroperoxy derivative is both more toxic and more phototoxic than the parent drug.
Rearranged diketone 2 is inactive in the dark but exhibits a photohemolytic activity comparable with that of the starting drug with both UV-A and UV-B. On the contrary, monoketone 3 does not demonstrate any significant photohemolytic effect under both irradiation conditions. The contrasting behavior of the two last products again indicates that the ketone function in C-20 is mainly responsible for the photohemolysis through its characteristic radicalic fragmentation. Indeed, the damage is observed when this function is present (as in starting drug 1 and in photoproduct 2, with a stronger effect with UV-B, where path b is predominant). No photohemolysis occurs when the isolated ketone is absent (as in photoproduct 3).
Because proteins and lipids are the main targets involved in membrane photodamage, the investigation was pursued by separately exploring these issues. Linoleic acid was chosen as a model system to test the ability of triamcinolone in photosensitizing peroxidation of unsaturated fatty acids. This was investigated by monitoring the absorbance increase at 233 nm due to the formation of conjugated dienic hydroperoxides as a function of the energy dose. As shown in Fig. 5, triamcinolone was able to induce lipid peroxidation under UV-B light. The extent of photoperoxidation was similar to that measured with well-known photosensitizers, such as phenothiazines (29), under the same conditions. Under UV-A irradiation the effect on the fatty acid was much smaller. Because, as seen above, ^sup 1^O^sub 2^ is unimportant, and this is consistent with a radicalic mechanism (Mechanism I; 30) for the peroxidation induced by 1.
Another possible mechanism of phototoxicity involves reaction of the sensitizer with RBC proteins; in particular, spectrin (the main protein present in the membrane bilayer of erythrocytes) can be cross-linked (31). No cross-linking of spectrin was observed when ghosts were irradiated with UV-A light in the presence of either triamcinolone or photoproducts 2-4. UV-B light was only slightly more effective in inducing spectrin cross-links than UV-A (12% of cross-linked spectrin under a UV-B dose as high as 5 J/cm^sup 2^), but at the doses used in the hemolysis experiments, i.e. 1 J/cm^sup 2^, the samples were quite similar to the irradiated controls (data not shown). Therefore, if radicals attacked proteins, this damage did not lead to cross-linking. This result was somehow unexpected, because radicals produced by other drugs (viz. phenothiazines) proved able to cross-link spectrin effectively (29,31). This behavior deserves further investigation to ascertain whether triamcinolone binds covalently to proteins on UV irradiation.
To evaluate the DNA-hreaking activity, pBR322 DNA was used as a model system. Under our conditions neither triamcinolone nor its photoproducts (50 µM) were able to induce DNA damage (single- and double-strand breaks) under UV-A light. Only at high UV-B doses (13 J/cm^sup 2^) was a small damage detected in terms of cleavage of supercoiled DNA to the open circular form (Fig. 6).
Finally, the phototoxicity of triamcinolone and its main photoproducts was assessed in 3T3 fibroblast cells using the MTT assay, carried out 24 h after irradiation. Cytotoxicity was not observed at any compound concentration when cells were not irradiated.
Figure 7 shows the phototoxicity in 3T3 cells. As it can be seen, triamcinolone demonstrated a low photocytotoxicity, the decrease in viability being almost constant increasing the compound dose (25, 50 and 100 µM). The photoproducts were also moderately toxic, except for product 2, which was highly cytotoxic at 100 µM concentration under high doses of UV-B radiation.
Therefore, the above triamcinolone cytotoxicity might be in part due to compound 2 formed during irradiation.
Summing up, photocytoxicity of 1 and its photoproducts was less conspicuous than photohemolysis. This result may in part be a consequence of experimental limitations because cell death was faster with UV-B than with UV-A even in the absence of these compounds (irradiated controls), whereas in the case of RBC the irradiation dose can be safely increased up to 1.5 J/cm^sup 2^, which is able to activate the drug. Another explanation may be found in the ability of fibroblasts to repair the damage induced by the drug because they bear elaborate ROS (reactive oxygenation species) defense mechanisms that are not present in the RBC.
CONCLUSIONS
A series of in vitro tests supported by photochemical studies allowed the rationalization of the observed UV phototoxicity of triamcinolone 16,17-acetonide. As in organic solvents (12), the drug undergoes extensive photolysis in water or PBS solutions-chosen for mimicking the physiological environment-and yields the same degradation products, which therefore have been considered in terms of phototoxic properties. The observed damage to the cell membrane is not predominantly due to oxygen activation but is rather related to organic radicals formed by Norrish Type-I homolytic fragmentation occurring at the C-20 ketone chromophore of the drug as well as to one of the stable photoproducts, the hydroperoxide (derivative 4), which is also active in the dark. A radical mechanism is supported by the effect of inhibitors on drug-induced photohemolysis and may involve primarily the lipids in the membrane, although firmer conclusions would require the knowledge of the subcellular localization of these compounds.
No clinical studies have been published showing that corticosteroids induce photosensitization. However, the data presented suggest that some photosensitizing effect in humans is possible, although lower than that exerted by other drugs such as quinolone antibiotics, anti-inflammatory drugs or phenothiazines. Therefore, prevention of exposure to sunlight or artificial UV-A sources during treatment with triamcinolone should be recommended. Moreover, because essential parts of the molecule (i.e. the cyclohexadienone ring and the 17 [beta]-ketol side chain) are deeply modified by UV light, the loss of therapeutic activity, both during storage and in vivo, cannot be ruled out.
Acknowledgements-This research was carried out in the frame of the program "Photoprocesses of Interest for Application," granted by the Universities of Padova and Pavia and by MIUR (Ministere dell'Istruzione, Universita c Ricerca) (Rome).
¶ Posted on the website on 25 September 2003
REFERENCES
1. Wilson, A. F. (1968) Drug treatment of asthma. In Fortschritte der Arzneimittelforschung, Vol. 28 (Edited by E. Jucker), pp. 122-125. Birkhauser Verlag, Basel, Switzerland.
2. Witkiewicz, I. M. and H. Neering (1981) Facial melanosis (melasma). Ned. Tijdschr. Genceskd. 125, 609-611.
3. Weiner, A. L. (1962) Intramuscular triamcinolone acetate therapy of dermatoses: preliminary report. Antibiot. Chemother. 12, 360-366.
4. Nierman, M. M. (1963) Triamcinolone acetate ointment in psoriasis and other dermatoses: a new method of topical application. Clin. Med. 70, 771-775.
5. Furlong, T., W. Leisenring, R. Storb, C. Anasetti, F. R. Appelbaum, P. A. Carpenter, H. J. Deeg, K. Doney, H. P. Kiem, R. A. Nash, J. E. Sanders, R. Witherspoon, D. Thompson and P. J. Martin (2002) Psoralen and ultraviolet A irradiation (PUVA) as therapy for steroid-resistant cutaneous acute graft-versus-host disease. Biol. Blood Marrow Transplant. 8, 206-212.
6. Oliveira, H. S., M. Goncalo and A. Figueiredo (2000) Photosensitivity to lomefloxacin. A clinical and photobiological study. Photodermatol. Photoimmunol. Photomed. 16, 116-120.
7. Gilchrest, B. A. and R. M. Goldwyn (1981) Topical chemotherapy of pigment abnormalities in surgical patients. Plast. Reconstr. Surg. 67, 435-439.
8. Fagart, J., B. Couette, A. Souque, E. Davioud, A. Marquet and M.-E. Rafeslin-Oblin (1998) Photoaffinity labeling of the human mineralo-corticoid receptor with steroids having a reactive group at position 3, 18 or 21. Biochim. Biophys. Acta 1388, 35-44.
9. Reisch, J., J. Zappel, A. Raghu and R. Rao (1995) Photochemical studies. Part 68. Solid state photochemical investigations of methylprednisolone, prednisolone and triamcinolone acetonide. Acta Pharm. Turc. 37, 13-17.
10. Takacs, M., N. Ekiz-Gucer, J. Reisch and A. Gergely-Zobin (1991) Lichtempfindlichkeit von Corticosteroiden in kristallinem Zustand. Photochemische Studien, 59. Mitt. Pharm. Acta Helv. 66, 137-140.
11. Di Pietra, A. M., V. Andrisano, R. Gotti and V. Cavrini (1996) Online post-column photochemical derivatization in liquid chromatographicdiode-array detection analysis of binary drug mixtures. J. Pharm. Biomed. Anal. 14, 1191-1199.
12. Ricci, A., E. Fasani, M. Mella and A. Albini (2001) Noncommunicating photoreaction paths in some pregna-1,4-diene-3,20-diones. J. Org. Chem. 66, 8086-8093.
13. Kraljic, I. and S. El Moshni (1978) A new method for the detection of singlet oxygen in aqueous solutions. Photochem. Pholobiol. 28, 577-581.
14. Pathak, M. A. and P. C. Joshi (1984) Production of active oxygen species by psoralens and ultraviolet radiation (320-400 nm). Biochim. Biophys. Acta 798, 115-126.
15. Valenzeno, D. P. and J. W. Trank (1985) Measurement of cell lysis by light scattering. Photochem. Photobiol. 42, 335-339.
16. Zhou, W. and D. E. Moore (1997) Photosensitizing activity of the anti-bacterial drugs sulfamethoxazole and trimethoprim. J. Photochem. Photobiol. B: Biol. 39, 63-72.
17. Steck, T. L. and J. A. Kant (1974) Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 31A, 172-180.
18. Peterson, G. L. (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83, 346-356.
19. Miolo, G., G. Viola, D. Vedaldi, F. Dall'Acqua, A. Fravolini, O. Tabarrini and V. Cecchetti (2002) In vitro phototoxic properties of new 6-desfluoro and 6-fluoro 8-methylquinolones. Toxicol. In Vitro 16, 683-693.
20. Ciulla, T. A., J. R. van Camp, E. Rosenfeld and I. E. Kochevar (1989) Photosensitization of single-strand breaks in pBR322 DNA by rose bengal. Photochem. Photobiol. 49, 293-298.
21. Caine, D. (1995) Photochemical rearrangements of 6/6 and 6/5-fused cross-conjugated cyclohexadienones. In Handbook of Photochemistry and Photobiology (Edited by W. M. Horspool and P.-S. Song), p. 701. CRC Press, Boca Raton, FL.
22. Schultz, A. G. (1995) Photorearrangement reactions of cross-conjugated cyclohcxadicnones. In Handbook of Photochemistry and Photobiology (Edited by W. M. Horspool and P.-S. Song), p. 685. CRC Press, Boca Raton.
23. Schaffner, K. and M. Demuth (1980) Photochemical rearrangement of conjugated cyclic dienones. In Rearrangements in the Ground and Excited State, Vol. 3 (Edited by P. De Mayo), pp. 281-345. Academic Press, New York.
24. Kraljic, I. and V. A. Sharpatyi (1978) Determination of singlet oxygen rate constants in aqueous solutions. Photochem. Photobiol. 28, 583-586.
25. Bordin, F., F. Baccichetti, C. Marzano, F. Carlassare, G. Miolo, A. Chilin and A. Guiotto (2000) DNA damage induced by 4,6,8,9-tetramethyl-2H-furo[2,3-h]quinolin-2-one, a new furocoumarin analog: photochemical mechanisms. Photochem. Photobiol. 71, 254-262.
26. Scaiano, J. C. and G. G. Wubbels (1981) Photosensitized dissociation of di-tert-butylperoxide. Energy transfer to a dissociative state. J. Am. Chem. Soc. 103, 640-645.
27. Engel, P. S., T. L. Woods and M. A. Page (1983) Quenching of excited triplet sensitizers by organic peroxides. J. Phys. Chem. 87, 10-13.
28. Tanielian, C. and R. Mechin (1997) Alkyl hydroperoxides as electron donors in photochemical reactions J. Photochem. Photobiol. A: Chem. 107, 291-293.
29. Elisei, F., G. Aloisi, L. Latterini, U. Mazzucato, G. Viola, G. Miolo, D. Vedaldi and F. Dall'Acqua (2002) Excited state properties and in vitro phototoxicity studies of three phenothiazine derivatives. Photochem. Photobiol. 75, 11-21.
30. Girolli, A. W. (1990) Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 51, 497-509.
31. Merville, M. P., J. Piette, J. Decuyper, C. M. Calberg-Bacq and A. Van De Vorst A (1983) Phototoxicity of phenothiazine derivatives. II. Photosensitised cross-linking of erythrocyte membrane proteins. Chem. Biol. Interact. 44, 275-287.
Giorgia Miolo*1, Andrea Ricci2, Sergio Caffieri1, Laura Levorato1, Elisa Fasani2 and Angelo Albini2
1 Department of Pharmaceutical Sciences, University of Padova, Padova, Italy and
2 Department of Organic Chemistry, University of Pavia, Pavia, Italy
Received 14 January 2003; accepted 10 August 2003
* To whom correspondence should be addressed at: Department of Pharmaceutical Sciences, Via Marzolo 5, I-35131 Padova, Italy. Fax: 39-049-827-5366; e-mail: giorgia.miolo@unipd.it
Copyright American Society of Photobiology Nov 2003
Provided by ProQuest Information and Learning Company. All rights Reserved