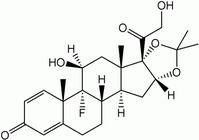
Study objectives: To determine if intrapleural administration of methylprednisolone acetate (MA) after therapeutic thoracentesis for symptomatic malignant pleural effusion improved time to repeat thoracentesis for symptom control, quality of life (QOL), and dyspnea.
Design: Double-blind, randomized, placebo-controlled trial.
Setting: A tertiary care cancer treatment center in Edmonton, AB, Canada.
Patient selection: Patients with symptomatic pleural effusions secondary to disseminated malignancy requiring therapeutic thoracentesis for symptom control.
Interventions: Sixty-seven patients underwent ultrasound-guided therapeutic thoracentesis for management of symptomatic malignant pleural effusion. Patients were randomly and blindly assigned to either 160 mg (8 mL) of MA or 8 mL of saline solution instilled into the pleural space. Patients were followed up for 6 weeks to determine the time to repeat therapeutic thoracentesis. All patients completed the Functional Assessment of Cancer Therapy-General (FACT-G) QOL questionnaire and a dyspnea visual analog scale (VAS) at baseline and again 2 weeks later.
Measurements and results: Thirty-three patients received MA, and 34 patients received placebo; baseline characteristics for the two groups were similar, apart from a slightly higher use of concurrent systemic therapy in the placebo group. At 6 weeks follow-up, 50% of MA-treated patients required repeat thoracentesis compared to 56% of placebo-treated patients (not significant [NS]). The mean of the individual FACT-G change scores (2 weeks--baseline) was similar in the two groups (NS). VAS scores improved for both groups over the 2-week period, but the mean change scores (2 weeks--baseline) were not statistically different.
Conclusion: Despite previous ease series describing benefit from intrapleural MA in malignant pleural effusion, this controlled study of intrapleural MA instillation did not delay reaccumulation of symptomatic pleural effusion compared to placebo, nor were differences in QOL or dyspnea observed. (CHEST 2003; 123:822-827)
Key words: corticosteroids; intrapleural; malignancy pleural effusion; thoracentesis
Abbreviations: FACT-G = Functional Assessment of Cancer Therapy-General., HRQOL = health-related quality of life; LDH = lactate dehydrogenase; MA = methylprednisolone acetate; NS = not significant; PWB = physical well-being; QOL = quality of life; VAS = visual analog scale
**********
Pleural effusion is common to many malignancies, including primary bronchogenic carcinoma, lymphoma, and metastatic disease from other primary sites such as ovary, breast, and stomach. When large volumes of fluid are present in the pleural space, patients commonly experience symptoms of shortness of breath, chest pain, cough, and anxiety. These symptoms frequently reduce the quality of life (QOL) of patients with cancer.
Steroids have been shown to be effective in dealing with third-space fluid accumulations secondary to malignancy. Results of a phase II trial performed by Mackey et al (1) using triamcinolone hexacetonide in the management of malignant ascites were positive in terms of increasing the time interval between therapeutic paracenteses and improving health-related QOL (HRQOL). Bartal et al (2) performed a nonblinded, phase II trial exploring the role of methylprednisolone acetate (MA) instillations into the pleural space of patients requiring thoracentesis for malignant pleural effusion. Six of 10 patients showed symptomatic benefit, and 3 patients demonstrated complete resolution of their effusion. The procedure was tolerated well and could be performed on an outpatient basis. To determine whether intrapleural corticosteroid instillation was effective in the management of malignant pleural effusions, we randomized patients in a double-blind, placebo-controlled fashion to receive either MA or placebo into the pleural space after thoracentesis.
MATERIALS AND METHODS
Study Design
This was a randomized, double-blind study of patients with symptomatic malignant pleural effusion comparing the intrapleural administration of MA, 160 mg, vs placebo after therapeutic thoracentesis. We conducted this trial at the Cross Cancer Institute, Edmonton, AB, Canada between July 1998 and July 2001. The protocol and consent form were approved by the Alberta Cancer Board Research Ethics Board. All patients provided written informed consent prior to enrollment. The primary objective of the study was to determine if instilling MA into the pleural space after therapeutic thoracentesis altered the mean time to reaccumulation of the effusion to the point that symptoms warranted another therapeutic drainage. Secondary objectives were to compare the HRQOL of patients of the two study arms using the Functional Assessment of Cancer Therapy-General (FACT-G) QOL measurement instrument, and to assess ongoing dyspnea using a visual analog scale (VAS). Toxicity assessments were also performed.
Patient Population
All patients were required to have symptomatic pleural effusion in the presence of disseminated malignancy, Eastern Cooperative Oncology Group performance status 0 to 3, life expectancy of at least 8 weeks, platelet count > 50,000/[micro]L, neutrophil count > 1,000/[micro]L, and the ability to provide informed, written consent. Patients could be enrolled at the development of their first symptomatic effusion or could be enrolled at time of recurrence of an effusion. Concurrent systemic and supportive therapies were allowed. Exclusion criteria included patients with uncontrolled congestive heart failure, active systemic infection, history of tuberculosis, previous pleurodesis to the involved hemithorax, and systemic corticosteroids received within 2 weeks prior to enrollment. Patients receiving any experimental drug therapies and pregnant or nursing women were excluded.
Treatment Administration and Evaluation
All patients were examined by a physician member of the clinical study team to confirm the presence of pleural effusion, and a chest radiograph was obtained. CBC, serum glucose, and serum total protein and lactate dehydrogenase (LDH) levels were obtained. Patients then underwent therapeutic thoracentesis under ultrasound guidance. Following thoracentesis and prior to removal of the catheter, either MA, 20 mg/mL, or saline solution placebo were instilled into the pleural space. In the active-treatment study arm, 160 mg of MA (8 mL) followed by 8 mL of saline solution flush were instilled. In the placebo-treatment study arm, 16 mL of saline solution was instilled, following which the catheter was removed. Because MA is a milky solution, drug or placebo administration was performed by a radiologist, who although not blinded to treatment assignment, was not involved in data analysis. Opaque syringes were used and hidden from the patient's view to maintain blinding. Postthoracentesis chest radiography was performed to rule out pneumothorax. Pleural fluid was sent for analysis including cell count, total protein, LDH, cytology, and culture.
Patients returned 2 weeks after thoracentesis for a repeat clinical examination, chest radiograph, FACT-G questionnaire, and completion of the dyspnea VAS. Timing of any interval thoracentesis was recorded. Patients were then contacted by telephone at the 6-week time point, and a complete chart review was obtained to determine the timing of any additional thoracenteses.
Outcome Measures
Dyspnea was measured at baseline and 2 weeks after treatment using a VAS. A review of the literature found that several dyspnea measurement scales exist, (3,4) but none are considered standard measurement tools. We chose a VAS for our study because it is reproducible, validated, and practical. HRQOL was measured at baseline and after treatment using the patient self-administered FACT-G instrument (Version 4.0). (5) This instrument consists of 27 items that measure QOL in four domains: emotional well-being (six items), functional well-being (seven items), physical well-being (PWB) [seven items], and social/family well-being (seven items). Each of the 27 items has a Likert-scale response with values ranging between 0 and 4. The four subscales corresponding to the four domains are scored using the method advocated by the Functional Assessment of Chronic Illness Therapy measurement system. (6) Individual subscale scores are also summed to form a total score. Higher subscale and total scores represent an improvement in well-being. The possible FACT-G total score ranges from zero to 108, and the possible PWB subscale score ranges from zero to 38.
Measurements for both the FACT-G total and PWB scores as well as the dyspnea VAS were obtained at baseline and at the 2-week follow-up. The group mean of the individual patient change scores (2 weeks--baseline) was analyzed for statistical difference using unpaired t tests.
Statistical Considerations and Sample Size
A review of the ultrasound logs at the Cross Cancer Institute from 1995 to 1998 indicated that approximately 60 patients per year were receiving thoracenteses under ultrasound guidance. The mean time between repeated thoracentesis for any given individual was 20 days (range, 1 to 90 days). This value was used to estimate the time to repeat thoracentesis for the control group. We defined a meaningful clinical benefit as an increase in the mean time to recurrence by 50% (ie, from 20 to 30 days between thoracenteses). A total of 66 patients, 33 patients in each study arm, was required to detect this difference using unpaired t tests with p = 0.05, power of 80%.
The trial was designed to compare intrapleural MA with placebo using time to repeat thoracentesis as primary end point, and changes in HRQOL and dyspnea as secondary end points. For the primary end point, Kaplan-Meier curves were generated and log-rank testing was used to determine if any statistically significant differences were found between the two groups for time to recurrence of symptomatic effusion requiring further thoracenteses.
For the secondary end points, the FACT-G questionnaires were scored and mean total score and PWB subscale scores were analyzed using unpaired t tests. Individual patient change scores were calculated taking the difference between the baseline and 2-week time points. The group mean of the individual change scores was compared using unpaired t tests. Dyspnea VAS measurements were analyzed in an identical fashion. All analyses were completed using SAS software (Version 8; SAS Institute; Cary, NC).
RESULTS
Patient Characteristics
Seventy patients were accrued between July 1998 and July 2001. Three patients were randomized but did not receive study medication; two patients withdrew consent, and one patient had a loculated effusion and fluid could not be removed. Analysis is restricted to the remaining 67 patients: 33 patients receiving intrapleural MA and 34 patients receiving placebo.
Demographic and clinical characteristics of the two groups were comparable. Data are presented in Table 1. Median age for the group was 62.5 years. There was a statistically significant difference between the groups with respect to the number of patients receiving active treatment compared to supportive care only at the time of enrollment, with 30 of 34 patients (88%) in the placebo-treated group receiving active anticancer treatment and 20 of 33 patients (61%) in the MA-treated group receiving active treatment (p = 0.009). It should be noted, however, that all patients enrolled on the study had metastatic and/or recurrent disease and none were considered curable. Furthermore, the overall number of patients pretreated with chemotherapy was not statistically different between groups (16 of 33 patients [48%] in the MA group; 16 of 34 patients [47%] receiving placebo), nor was there any difference in the mean number of regimens received for patients who were pretreated (2.1 regimens for the MA group; 1.9 regimens for the placebo group). Similar nonsignificant results were seen for prior hormonal therapy between groups (Table 1).
Table 2 demonstrates the frequency of various cancers in the population studied. Breast cancer was the most frequently noted malignancy. This may be in part due to referral patterns, as two of the investigators work predominantly in breast cancer care. Lung cancer is relatively underrepresented when one considers the number of patients with this illness and the frequency of lung cancer-associated pleural effusions. This is because the majority of our patients with lung cancer and pleural effusions had received a pleurodesis prior to being referred to our institution.
Table 1 also depicts the biochemical results from thoracentesis for the two groups. The median volume of pleural fluid removed was 1,250 mL for the MA group and 1,000 mL for the placebo group (p = 0.04). Positive malignant cytology results were seen in 25 patients (76%) in the MA group and 24 patients (71%) in the placebo group. No differences were noted in the biochemical composition of the fluid between groups. Ratios of pleural fluid to serum LDH and total protein confirm that these fluids are exudates, as one would expect from malignant effusions.
Efficacy
Results for the primary end point, time to recurrence of pleural effusion symptomatic enough to require thoracentesis, is shown as a Kaplan-Meier plot in Figure 1. Sixteen patients in the MA group and 15 patients in the placebo group required another thoracentesis by the 42-day follow-up point of the protocol. Seventeen patients in the MA group and 19 patients in the placebo group never required another thoracentesis for symptom control during the study. By the end of the 42-day follow-up period, 48.5% of MA-treated patients required repeat thoracentesis. In the placebo group, at the 42-day follow-up point, 44.1% of patients had required a repeat thoracentesis.
[FIGURE 1 OMITTED]
HRQOL
QOL questionnaires were available for analysis from all patients at baseline. Thirty of 33 patients in the MA group and 33 of 34 patients in the placebo group completed the 2-week questionnaire. There were no missing items on completed questionnaires.
QOL and dyspnea data are presented in Table 3. At baseline, the QOL score for the two groups was similar, with a total score of 59.5 in the MA group as compared to 54.7 in the placebo group. Analysis at the 2-week point showed total scores of 60.4 and 57.1 in the MA and placebo groups, respectively (not significant [NS]). Statistical comparison of the mean of the individual change scores between baseline and the 2-week point for the two groups was done to determine if any significant changes in QOL occurred between the two groups. The mean of the change scores for the MA group was 0.94 and for placebo was 2.46 (NS). A similar analysis was done for the PWB subsection of the FACT-G and again demonstrated no difference (data not shown).
The baseline dyspnea VAS was similar, with the MA group rating their dyspnea at 7.0/10 and the placebo group at 6.7/10 (NS). At week 2, both groups indicated that their dyspnea had improved, as was expected since all patients underwent therapeutic thoracentesis. The mean of the change scores, however, did not differ between groups, with the MA group showing a 2.2-cm improvement and the placebo group showing a 1.5-cm improvement (NS).
DISCUSSION
The pleural space is a potential space through which pleural fluid passes at a rate of 5 to 10 L/d. (7) Should an imbalance between production and reabsorption of fluid occur, fluid will accumulate. In malignancy, this occurs either because of impaired outflow of pleural fluid due to lymphatic obstruction and/or increased production of pleural fluid due to the presence of pleural-based metastases. (7,8) In the latter case, malignant cells may be found in the pleural fluid; however, several series have shown that cytology results are positive only in 60% of cases. (7)
Malignant pleural effusion is a common problem among cancer patients. Breast, lung, stomach, and ovarian cancers as well as lymphoma are prone to this complication. There is significant morbidity to patients due to troubling pulmonary symptomatology, and QOL is often poor. Standard treatment of malignant pleural effusion incorporates both systemic and local measures? Systemic management may include IV chemotherapy, hormonal therapy, and newer biologics. Local measures commonly include the following: (1) periodic thoracentesis with drainage of fluid as required for symptom control, (2) pleurodesis of the pleural space after drainage of the effusion to prevent its recurrence, and (3) intrapleural chemotherapy or biologics. (10,11) Pleurodesis with talc after chest tube insertion has often been a favored approach to try and minimize recurrence of fluid and offer patients prolonged relief from symptoms. (10) Unfortunately, it is not always effective, there are complications from the procedure itself, and it is painful.
MA is a synthetic steroid that is often used for intra-articular injection in patients with arthritis. Dosages from 20 to 80 mg per injection have been used depending on the size of the joint being treated. Treatments may be repeated as clinically indicated. Side effects of local injection tend to be minimal and self-limited and usually relate to transient discomfort at the site of injection. Early data had suggested that instillation of MA into the pleural space after therapeutic thoracentesis may reduce the time to recurrence of fluid. Bartal et al (2) published the results of their work using intrapleural MA in 1991. In their trial, they subjected patients to multiple, frequently spaced thoracenteses in order to drain the pleural space. At each thoracentesis, MA was instilled, initially 80 mg then increasing to 160 mg, with the mean cumulative amount of steroid administered per patient being 420 mg. Six of 10 patients were reported to have benefited, 3 patients with complete resolution of their effusion.
To determine the value of intrapleural corticosteroid instillation in malignant pleural effusion, we performed a larger, phase III trial. In our trial, we opted to perform a single-time thoracentesis followed by 160 mg of intrapleural MA, the highest dose used in the study by Bartal et al. (2) Our results indicate that steroid instillation with MA after therapeutic thoracentesis was not effective in delaying time to pleural effusion recurrence and need for another thoracentesis for symptom control in this patient population. Sixteen patients in the MA group and 15 patients in the placebo group required repeat thoracentesis for symptom control during the follow-up period, resulting in 48.5% and 44.1% of patients requiring repeat thoracentesis by the end of the 42 day follow-up period, respectively. HRQOL was similarly unaffected by the MA instillations, with no statistical difference in VAS dyspnea scores or FACT-G questionnaire scores between groups. Our inability to detect any positive effect suggests that other administration schedules are unlikely to materially benefit patients.
Despite randomization, there were imbalances between treatment groups. The placebo group had more patients receiving active anticancer therapy (30 of 34 patients, 88%) compared to the intervention group (20 of 33 patients, 61%) at the time of study enrollment. However, the majority of patients in either group (22 of 33 patients, 66.7%, in the MA group; 23 of 34 patients, 67.6%, in the placebo group) had been exposed to prior systemic therapy at the time of enrollment, with a mean number of treatment regimens of 2.3 for MA and 2.1 for placebo. The breakdown of systemic regimens into chemotherapy and hormonal therapy was not statistically different between groups. Furthermore, all patients enrolled had metastatic or recurrent malignancies and were considered incurable. As control of malignant pleural effusions in this patient population with either chemotherapy or hormonal treatment is generally unsuccessful, it is unlikely that this 27% difference between groups would have had a substantial effect in controlling pleural effusions in this heavily pretreated population. Additionally, the minor difference (250 mL) in median volume of pleural fluid removed at thoracentesis in the MA group would not be expected to negate a clinically relevant effect of MA instillation.
Our secondary end points of HRQOL and dyspnea were similarly unchanged by the intervention. Since pleural effusion can impact very negatively on a person's ability to perform activities of daily living, we felt an assessment of QOL and dyspnea would be essential. An analysis of the dyspnea VAS scores indicates that patients in both groups experienced an improvement in their dyspnea at week 2 compared to baseline. This is not surprising given the fact that a therapeutic thoracentesis was performed. However, the magnitude of benefit was not statistically different between groups, suggesting that the benefit was only from the thoracentesis and not from the MA that was instilled into the pleural space. At baseline, the QOL scores as measured by the FACT-G questionnaire were similar between groups. When QOL was measured again at 2 weeks, there were essentially no changes compared to baseline scores, and the mean of the change scores for patients in either treatment group were similar, indicating no significant changes in QOL between treatment arms. We performed a subanalysis looking only at the PWB subset of questions of the FACT-G, since that is the domain that we believed would most likely change if there were a positive effect from the steroids. Again, no improvement was noted in the MA study arm as compared to placebo study arm.
We conclude that MA instillation in malignant pleural effusions is not effective in reducing the requirement for repeat thoracentesis and does not improve dyspnea or HRQOL. Its use cannot be recommended outside the setting of a clinical trial.
ACKNOWLEDGMENT: The authors thank Colleen Sorokan for assistance in data entry, John Hanson for statistical analyses, and Mary Bums for assistance in manuscript preparation. We also thank Pharmacia & Upjohn for providing the methylprednisolone acetate used in the study.
REFERENCES
(1) Mackey JR, Wood L, Nabholtz J, et al. A phase II trial of triamcinolone hexacetonide for symptomatic recurrent malignant ascites. J Pain Symptom Manage 2000; 19:193-199
(2) Bartal AH, Gazitt Y, Zidan G, et al. Clinical and flow cytometry characteristics of malignant pleural effusions in patients after intracavitary administration of methylprednisolone acetate. Cancer 1991; 67:3136-3140
(3) McGavin CR, Artvinli M, Naoe H, et al. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. BMJ 1978; 2:241-243
(4) Farncombe M. Dyspnea: assessment and treatment. Support Care Cancer 1997; 5:94-99
(5) Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993; 11:570-579
(6) Cella D. FACIT manual: manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System, Version 4, November 1997.
(7) Fenton KN, Richardson JD. Diagnosis and management of malignant pleural effusions. Am J Surg 1995; 170:69-74
(8) Light RW. Diagnostic principles in pleural disease. Eur Respir J 1997; 10:476-481
(9) Sahn SA. Malignant pleural effusions. Clin Chest Med 1985; 6:113-125
(10) Walker-Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994; 120:56-64
(11) Rauthe G, Sistermanns J. Recombinant tumour necrosis factor in the local therapy of malignant pleural effusion. Eur J Cancer 1997; 33:226-233
Scott A. North, MD; Heather-Jane Au, MD, MPH; Steven B. Halls, MD; Linda Tkachuk, RN; and John R. Mackey, MD
* From the Department of Oncology, University of Alberta, Edmonton, AB, Canada.
Manuscript received April 24, 2002; revision accepted September 13, 2002.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: permissions@chestnet.org).
Correspondence to: Scott North, MD, Cross Cancer Institute, 11560 University Ave, Edmonton, AB, Canada T6G 1Z2; e-mail: scottnor@cancerboard.ab.ca
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group