Study objectives: A blunted hypoxic ventilatory response (HVR) has been observed in some sufferers of high-altitude pulmonary edema (HAPE), and was proposed as a potential mechanism in its pathogenesis. Tyrosine hydroxylase (TH) is a rate-limiting enzyme in the carotid body responding to hypoxia to synthesize dopamine neurotransmitter to heighten ventilation. The association of constitutional susceptibility to HAPE regarding the blunted HVR aspect with polymorphisms of the TH gene was examined.
Design: A cross-sectional case control study.
Setting: Shinshu University Hospital, Matsumoto, Japan.
Participants: Forty-three subjects with a history of HAPE (HAPE group) and 51 healthy climbers without a history of HAPE (control group).
Measurements: The (TCAT)n tetranucleotide microsatellite repeats within intron 1 and Met81Val variant in exon 2 of the TH gene were investigated by polymerase chain reaction following either direct sequencing or restriction fragment length polymorphism. The HVR in 21 subjects among the HAPE group was also measured.
Results: No significant frequency differences could be found in terms of either of the two polymorphisms between the HAPE and control groups. Meanwhile, no relationships were observed between the HVR values of HAPE subjects and the individual alleles in both polymorphisms of the TH gene.
Conclusion: The genetic susceptibility of HAPE, specifically the blunted HVR in HAPE, is probably not associated with the mutations of the TH gene, implying that these two polymorphisms may not be a sufficient genetic marker for predicting a predisposition to the susceptibility to HAPE.
Key words: carotid body; high-altitude pulmonary edema; hypoxic ventilatory response; polymorphism; tyrosine hydroxylase gene
Abbreviations: CB = carotid body; HAPE = high-altitude pulmonary edema; HAPE-r = high-altitude pulmonary edema resistant; HAPE-s = high-altitude pulmonary edema susceptible; HIF = hypoxia-inducible factor; HVR = hypoxic ventilator response; Met = methionine; PCR = polymerase chain reaction; Sa[O.sub.2] = arterial oxygen saturation; TH = tyrosine hydroxylase; Val = valine; VE = minute ventilation
**********
High-altitude pulmonary edema (HAPE) is an acute respiratory distress that occurs in many unacclimatized individuals who are exposed to altitudes > 2,500 m. (1) The observation of the existence of HAPE-resistant (HAPE-r) and HAPE-susceptible (HAPE-s) subjects does suggest the possible prediction of a genetic and/or acquired predisposition in susceptible individuals. (2,3)
A series of studies hypothesized that the blunted hypoxic ventilatory response (HVR) contributed to the pathogenesis of HAPE. (4-6) HAPE-s subjects more frequently show low HVR than their control counterparts; alternatively, low HVR is disadvantageous to HAPE-s subjects at high altitude. Measuring HVR was once considered as a noninvasive means to identify the susceptibility to HAPE. (5,6) However, the direct genetic evidence to support this hypothesis has been absent until now. (7-9)
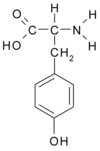
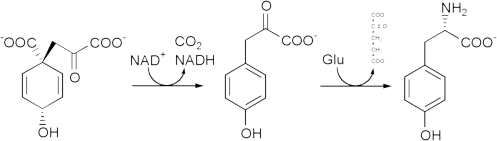
The peripheral chemoreceptor for hypoxia is located in the carotid body (CB). (10) When CB glomus cells are stimulated by hypoxia, the cells depolarize and release catecholaminergic neurotransmitter dopamine that subsequently emanates neuronal signals to a higher ventilatory center resulting in hyperventilation. (11) Tyrosine hydroxylase (TH) is a ratelimiting enzyme in the catecholamine synthesis pathway for producing dopamine in CB. (8) The gene encoding human TH is assigned on chromosome 11p15 and is comprised of 14 exons and 13 introns distributed over 8.5 kilobases. (12) Crucially, (TCAT)n tetranucleotide repeats, a microsatellite marker, was found to be located in the first intron of the human TH gene, and at least five individual alleles have been identified in a population of randomly selected individuals. (13) In addition, a common amino acid variant swapping valine (Val) for methionine (Met) at codon 81 was recently described in exon 2 of the TH gene. (14) Studies have shown that the polymorphisms of the TH gene are positively associated with turnover of the catecholamine synthesis pathway (15) and diseases involving catecholaminergic disturbances and dysfunctions, such as essential hypertension (16) and schizophrenia. (17) Considering that sympathetic activity is increased in humans during acclimation to high altitude, (18) we hypothesized that the lower HVR in HAPE-s subjects was possibly related with a neurotransmission blunting due to insufficient production of dopamine in CB based on the TH gene mutation. To clarify this proposition, we examined the informative tetranucleotide (TCAT)n microsatellite repeats marker and Met81Val variant of the TH gene in HAPE-s and HAPE-r populations in a case-control model. Furthermore, we also measured the HVR values within the HAPE-s group in order to seek the correlation of the blunted HVR in HAPE with the TH gene polymorphisms.
MATERIALS AND METHODS
Study Population
We collected the venous blood samples from 43 subjects with histories of HAPE (HAPE-s cohort) and 51 healthy volunteers without histories of HAPE (HAPE-r cohort). All subjects were unrelated natives of Japan, born and residing at lower altitudes than our institute in Matsumoto City (610 m above sea level). Written informed consent was obtained from every subject after a full explanation of the study that was approved by the Ethics Committee of the Shinshu University.
The subjects with histories of HAPE consisted of 40 males and 3 females (age range, 15 to 75 years; average age, 31.3 years). They were affected by HAPE while climbing the Japan Alps, and were transported to our hospital by helicopter from July 1979 to August 2000. The altitude at the onset of HAPE ranged from 2,758 to 3,190 m above sea level. We diagnosed HAPE on the basis of the following diagnostic criteria (2): onset at high altitude of the typical symptoms, including cough and dyspnea at rest; absence of infection; presence of pulmonary rales, and cyanosis; disappearance of symptoms and signs within 3 days of the start of treatment with bed rest and supplemental oxygen; and chest radiographic infiltrates consistent with pulmonary edema. All subjects with HAPE met all criteria at the onset of the disorder and recovered promptly with hospitalization. They were all in good healthy condition at the time of the present study.
The HAPE-r cohort consisted of 43 men and 8 women (age range, 18 to 62 years; average age, 38.6 years). All were elite mountain climbers affiliated with alpine clubs in Japan. Their average number of exposures > 2,800 m was > 100 times. None reported any history of medical problems related to altitude or cardiopulmonary disorders in a self-made questionnaire that contented with the components of Lake Louise Score. (19)
Identification of the (TCAT)n Tetranucleotide Microsatellite Repeats and Met81Val Variant in the TH Gene
Genomic DNA was extracted from peripheral blood by phenol extraction of sodium dodecyl sulfate-lysed and proteinase K-treated cells as a standard method. (20) The five alleles of (TCAT)n tetranucleotide repeats were typed by polymerase chain reaction (PCR) following direct sequencing (ABI 377 DNA sequencer; PE Applied Biosystems; Norwalk, CT). In addition, the Met and Val alleles of the Met81Val variant were examined by PCR following restriction fragment length polymorphism with restriction endonucleotide enzyme NlaIII. The genome sequences of the corresponding primers and PCR conditions were accorded as in a previous study. (16) All alleles were assigned by one single observer.
HVR Measurement
The HVR was measured during the hospitalization of the patients. HVR measurements were performed in a total of 21 patients with HAPE during a limited period for a research project concerning the blunted HVR in patients with HAPE in our institute. We evaluated the HVR by the progressive isocapnic hypoxic test, (5) using the slope of linear regression between minute ventilation (VE) and arterial oxygen saturation (Sa[O.sub.2]). This slope was termed VE/Sa[O.sub.2]. (5)
Statistical Analysis
The exact test of Hardy-Weinberg equilibrium for multiple alleles was performed by the Markov chain method within the GENEPOP software package (Curtin University of Technology; Bentley, Australia). (21) The Markov chain method has the advantage of obtaining a complete enumeration for testing Hardy-Weinberg equilibrium in cases where the number of alleles and the sample size are small. The frequencies of each genotype as well as each allele of either (TCAT)n repeats or the Met81Val variant between HAPE-s and HAPE-r groups were assessed by the [chi square] test (2 x 2 contingency table).
HVR values of the 21 HAPE-s subjects were expressed as means [+ or -] SEM. We defined five subsets by the five alleles in (TCAT)n repeats and two subsets by the two alleles in Met81Val variant, respectively, within the HAPE-s group. The mean HVR value among the five subsets concerned the (TCAT)n repeats was analyzed by one-way analysis of variance software (StatView version 5.0; SAS Institute; Cary, NC). In the meantime, the mean HVR value between the two subsets of the Met81Val variant was compared by unpaired Student t test. All p values < 0.05 were considered statistically significant for the [chi square] test, analysis of variance, and unpaired Student t test.
RESULTS
Frequencies of the Polymorphisms of the TH Gene
The genotypic and the allelic frequencies concerning the (TCAT)n tetranucleotide repeats or the Met81Val variant in the TH gene in each group are summarized in Tables 1, 2, respectively. Five alleles of (TCAT)n tetranucleotide repeats were positively identified in 42 HAPE-s and 49 HAPE-r individuals, designated as A, B, C, D, and E with relative sizes of 311, 315, 319, 323, and 327 base-pairs, respectively. No significant differences were found between HAPE-s and HAPE-r cohorts in the genotypic as well as allelic frequencies in the (TCAT)n tetranucleotide repeats polymorphism (Table 1).
Two alleles for the Met81Val variant were identified in all the 43 HAPE-s and 51 HAPE-r individuals, denoted as Met and Val alleles. Similarly, no significant differences were found between the two groups in the genotypic as well as allelic frequencies in the Met81Val variant (Table 2).
HVR
The mean value of HVR in 21 HAPE-s subjects was - 0.38 [+ or -] 0.04 L/min/%, significantly lower than the well-known normal range reported elsewhere. (4-6) We could not find any significant differences of the HVR values among each of the five allelic subsets regarding (TCAT)n repeats, as well as between the two allelic subsets in terms of the Met81Val variant in TH gene (Table 3).
DISCUSSION
In the present study, we compared the genotypes regarding the polymorphisms for TH gene in HAPE-s and HAPE-r individuals and found no differences between the two groups. A limitation of our study might have been the relatively low number of 43 investigated HAPE-s subjects. However, the current number of HAPE-s subjects is the maximum we could be obtained in our institute, since the incidence of HAPE in moderate Japan Alps is considerably low. (22) Fortunately, the Japanese population is a considerably homogenous race, so the population stratification based on ethnicity is less likely to occur in the present Japanese case-control study. Additionally, we used the Markov chain method within the GENEPOP software package that has a special advantage for small sample size. Therefore, even though the sample size of the present study is relatively small compared with other case-control reports worldwide, the reliability and validity of our findings could be reasonably acceptable since all the subjects are Japanese.
It has long been observed and demonstrated that HAPE-s individuals showed exaggerated hypoxemia with relative hypoventilation during the early period of high-altitude exposure compared with their corresponding HAPE-r control counterparts. (4-6) HVR, a slope of linear regression between VE and Sa[O.sub.2], was applied to describe such phenomena and evaluate the hypoxic ventilatory drive in HAPE-s individuals. (5,6) HVR was once considered as an important physiologic marker to predict the susceptibility to HAPE. (5,6) However, HVR shows considerable inter-individual variation (23,24) influenced by many factors including genetic and environmental. (25) The blunted HVR trait was uncertain in HAPE-s subjects because some expressed normal or even elevated HVR and still had HAPE, whereas others with markedly attenuated HVR remained asymptomatic. (4-6,26) These studies suggested that the low HVR played a permissive rather than causative role in the pathogenesis of HAPE. (4,26) Doubts have been raised with regard to the importance of blunted HVR in the pathogenesis of HAPE and whether it is a credible physiologic marker for predisposing susceptibility to HAPE. The evidence concerning the polymorphism of candidate genes involving HVR was absent until our current identification, in which we found no association between either the (TCAT)n tetranucleotide repeats or the Met81Val variant of the TH gene with HAPE, although the measured HVR in HAPE-s subjects was apparently low. Therefore, it is speculated that blunted HVR is probably one of the exacerbating factors in the progression of HAPE other than the most important trigger factor.
The TH is a key enzyme regulating the rate-limiting catecholamine synthetic pathway for producing dopamine, which has been suggested to be critical in the ventilatory response to hypoxia. (8,11) The TH gene expression in CB is up-regulated via increasing the rate of messenger RNA transcription and enhancing its stability under hypoxia, following the TH activation. (8) However, we failed to find any associations between these two polymorphisms in the TH gene with the blunted HVR in HAPE-s subjects. This negative association suggests that these polymorphisms do not significantly contribute to the susceptibility of HAPE, specifically the blunted HVR in HAPE-s individuals. Actually, how extensive the genetic and nongenetic factors impact on the inheritability of ventilation and HVR traits remains to be clarified. Using an admixture-based approach, Curran et al (27) compared ventilation and HVR in the offspring of Tibetan and Han (Chinese) parents (Tibetan-Han) and found that Tibetan-Han were similar to Tibetans in terms of their ventilation but resembled Han with respect to HVR, suggesting that different genetic factors influence ventilation and HVR in these high-altitude residents. Tibetan and Aymara high-altitude native populations showed both interpopulation and intrapopulation genetic variations in ventilation and HVR traits, with more intrapopulation variation in Tibetans than Aymaras, implying potential environmental factors in determining the characteristics of ventilation and HVR traits. (28) Moreover, it was observed that high-altitude natives residing at sea level, even previously at high altitude for > 20 years, did not maintain their previous blunted hypoxic responses, indicating this blunting was reversible on migration to sea level with high environmental determination. (29) In such viewpoints, our current polymorphic association study reveals that the blunted HVR in HAPE is more likely controlled by multinongenetic factors, implying that the blunted HVR may not be a specific creditable marker for the susceptibility to HAPE.
However, the TH enzyme is only one of the aspects in the mechanism of HVR in CB. Oxygen sensing in glomus cells is critical for the regulation of ventilation in CB. Progress has clarified that multiple gene-triggering mechanisms interact to contribute to oxygen sensing in glomus cells to express the HVR traits. (7) Testing a single genetic variant for an association with a single phenotype base statistical threshold for significance on a multiple-mechanic disease should be avoided. (30) Another point is that in the present study we did not examine other fragments in the TH gene that might play important roles in the transcriptional regulation of TH gene expression responding to hypoxia in glomus cells, for example, a short fragment of the proximal promoter that contains hypoxia-inducible factor (HIF)-1 binding site sequences. (9) Mutation within this region can abolish the binding of HIF-1 to prevent the regulation of TH messenger RNA at the transcriptional and posttranscriptional level. Therefore, the only conclusion that could be resulted from the present study is that there are no direct relationships between the current examined TH gene polymorphisms with HAPE and the blunted HVR in HAPE-s as well. Further studies are needed to elucidate whether there are any other polymorphisms in some fragments (for example, the HIF-1 binding site .sequences) in TH gene, which may possibly associate with HAPE-s or the blunted HVR in HAPE, and whether there are any other linkage candidate genes responsible for TH gene expression to hypoxia.
In summary, no significant association regarding the (TCAT)n tetranucleotide repeats and Met81Val variant polymorphisms of the TH gene was found between HAPE-s and HAPE-r. Moreover, no significant correlation was observed regarding the blunted HVR value in HAPE-s with any allele in both polymorphisms of the TH gene. The current mutations of the TH gene probably do not impact on the pathogenesis of HAPE, specifically on the blunted HVR in HAPE-s individuals, suggesting that these two polymorphisms may not be sufficient genetic markers for predisposing to the susceptibility to HAPE.
REFERENCES
(1) Hackett PH, Roach RC. High-altitude illness. N Engl J Med 2001; 345:107-114
(2) Hultgren HN, Marticorena EA. High altitude pulmonary edema: epidemiological observations in Peru. Chest 1978; 74:372-376
(3) Hanaoka M, Kubo K, Yamazaki Y, et al. Association of high-altitude pulmonary edema with the major histocompatibility complex. Circulation 1998; 97:1124-1128
(4) Hackett PH, Roach RC, Schoene RB, et al. Abnormal control of ventilation in high-altitude pulmonary edema. J Appl Physiol 1988; 64:1268-1272
(5) Matsuzawa Y, Fujimoto K, Kobayashi T, et al. Blunted hypoxic ventilatory drive in subjects susceptible to high-altitude pulmonary edema. J Appl Physiol 1989; 66:1152-1157
(6) Hohenhaus E, Paul A, McCullough RE, et al. Ventilatory and pulmonary vascular response to hypoxia and susceptibility to high altitude pulmonary edema. Eur Respir J 1995; 8:1825-1833
(7) Lahiri S, Rozanov C, Cherniack NS. Altered structure and function of the carotid body at high altitude and associated chemoreflexes. High Alt Med Biol 2000; 1:63-67
(8) Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, et al. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem 1992; 58:1538-1546
(9) Norris ML, Millhorn DE. Hypoxia-induced protein binding to [O.sub.2]-responsive sequences on the tyrosine hydroxylase gene. J Biol Chem 1995; 270:23774-23779
(10) Barnard P, Andronikou S, Pokorski M, et al. Time-dependent effect of hypoxia on carotid body chemosensory function. J Appl Physiol 1987; 63:685-691
(11) Buerk DG, Osanai S, Mokashi A, et al. Dopamine, sensory discharge, and stimulus interaction with C[O.sub.2] and [O.sub.2] in eat carotid body. J Appl Physiol 1998; 85:1719-1726
(12) Craig SP, Buckle VJ, Lamouroux A, et al. Location of the human tyrosine hydroxylase gene to 11p15: gene duplication and evolution of metabolic pathways. Cytogenet Cell Genet 1986; 42:29-32
(13) Polymeropoulos MH, Xiao H, Rath DS, et al. Tetranucleotide repeat polymorphism at the human tyrosine hydroxylase gene (TH) [abstract]. Nucleic Acids Res 1991; 19:3753
(14) Ludecke B, Bartholome K. Frequent sequence variant in the human tyrosine hydroxylase gene [abstract]. Hum Genet 1995; 95:716
(15) Wei J, Ramchand CN, Hemmings GP. Possible association :of catecholamine turnover with the polymorphic (TCAT)n repeat in the first intron of the human tyrosine hydroxylase gene. Life Sci 1997; 61:1341-1347
(16) Sharma P, Hingorani A, Jia H, et al. Positive association of tyrosine hydroxylase microsatellite markers to essential hypertension, Hypertension 1998; 32:676-682
(17) Wei J, Ramchand CN, Hemmings GP. Association of polymorphic VNTR region in the first intron of the human TH gene with disturbances of the catecholamine pathway in schizophrenia. Psychiatr Genet 1995; 5:83-88
(18) Duplain H, Vollenweider L, Delabays A, et al. Augmented sympathetic activation during short-term hypoxia and high-altitude exposure in subjects susceptible to high-altitude pulmonary edema. Circulation 1999; 99:1713-1718
(19) Hackett P, Oelz O. The Lake Louise Consensus of the definition and quantification of altitude illness. In: Sutton J, Coates G, Houston C, eds. Hypoxia and mountain medicine. Burlington, VT: Queen City Printers, 1992; 327-330
(20) Inoko H, Ando A, Ito M, et al. Southern hybridization analysis of DNA polymorphism in the HLA-D region. Hum Immunol 1986; 16:304-313
(21) Guo SW, Thompson EA. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 1992; 48:361-372
(22) Kobayashi T, Koyama S, Kubo K, et al. Clinical features of patients with high-altitude pulmonary edema in Japan. Chest 1987; 92:814-821
(23) Berezovskii VA, Zhigailo TL. Individual differences in hypoxic responses at high altitude in the mountains. Hum Physiol 1979; 5:74-78
(24) Vizek M, Pickett CK, Weil JV. Interindividual variation in hypoxic ventilatory response: potential role of carotid body. J Appl Physiol 1987; 63:1884-1889
(25) Lahiri S, DeLaney RG, Brody JS, et al. Relative role of environmental and genetic factors in respiratory adaptation to high altitude. Nature 1976; 261:133-135
(26) Selland MA, Stelnzer TJ, Stevens T, et al. Pulmonary function and hypoxic ventilatory response in subjects susceptible to high-altitude pulmonary edema. Chest 1993; 103:111-116
(27) Curran LS, Zhuang J, Sun SF, et al. Ventilation and hypoxic responsiveness in Chinese-Tibetan residents at 3,658 m. J Appl Physiol 1997; 83:2098-2104
(28) Beall CM, Strohl KP, Blangero J, et al. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol 1997; 104:427-447
(29) Vargas M, Leon-Velarde F, Monge CC, et al. Similar hypoxic ventilatory response in sea-level natives and high-altitude Andean natives living at sea level. J Appl Physiol 1998; 84:1024-1029
(30) Altshuler D, Kruglyak L, Lander E. Genetic polymorphisms and diseases [letter]. N Engl J Med 1998; 338:1626
* From the First Department of Medicine (Drs. Hanaoka, Droma, Hotta, Matsuzawa, Kobayashi, and Kubo) and Department of Legal Medicine (Dr. Ota), Shinshu University School of Medicine, Matsumoto, Japan.
This study was supported partly by a Grant-in-Aid for Scientific Research (B), No. 13470126, from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Manuscript received March 5, 2002; revision accepted July 23, 2002.
Correspondence to: Masayuki Hanaoka, MD, First Department of Internal Medicine, Shinshu University School of Medicine, 3-1-1 Asahi, Matsumoto 390-8621, Japan; e-mail: masayuki@ hsp.md.shinshu-u.ac.jp
COPYRIGHT 2003 American College of Chest Physicians
COPYRIGHT 2003 Gale Group