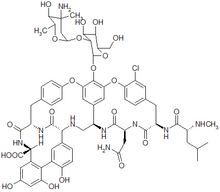
Vancomycin
Vancomycin is an antibiotic used in the prophylaxis and treatment of infections caused by Gram-positive bacteria. It is a branched tricyclic glycosylated nonribosomal peptide produced by the fermentation of the Actinobacteria species Amycolatopsis orientalis (formerly Nocardia orientalis). more...
It is often reserved as the "drug of last resort", used only after treatment with other antibiotics had failed. With the increasing prevalence of antibiotic resistant-bacteria, vancomycin has increasingly become a first line therapy when faced with Staphylococcus aureus infections in a patient where antibiotic resistance can reasonably be anticipated.
Vancomycin hydrochloride has been developed by Eli Lilly under the trade name Vancocin®. The patent expired in the early 1980s and generic versions of the drug are now available internationally under various trade names.
In 2004, Eli Lilly licensed Vancocin to ViroPharma Incorporated.
Mechanism of action
Vancomycin acts by inhibiting proper cell wall synthesis in Gram-positive bacteria. The mechanism inhibited, and various factors related to entering the outer membrane of Gram-negative organisms mean that vancomycin is not active against Gram-negative bacteria.
Specifically, vancomycin prevents incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits from being incorporated into the peptidoglycan matrix; which forms the major structural component of Gram-positive cell walls.
The large hydrophilic molecule is able to form hydrogen bond interactions with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides. Normally this is a five-point interaction. This binding of vancomycin to the D-Ala-D-Ala prevents the incorporation of the NAM/NAG-peptide subunits into the peptidoglycan matrix.
Vancomycin has two chemically distinct rotamers owing to the rotational restriction of the chlorotyrosine residue (on the right hand side of the figure). The form present in the drug is the thermodynamically more stable conformer, and, importantly, has more potent activity. Vancomycin also displays atropisomerism.
Therapeutic considerations
Toxicity
Vancomycin has traditionally been considered a nephrotoxic and ototoxic drug, based on observations by early investigators of elevated serum levels in renally impaired patients who had experienced ototoxicity, and subsequently through case reports in the medical literature. However, as the use of vancomycin increased with the spread of MRSA beginning in the seventies, it was recognized that the previously reported rates of toxicity were not being observed. This was attributed to the removal of the impurities present in the earlier formulation of the drug, although those impurities were not specifically tested for toxicity.
Nephrotoxicity
Subsequent reviews of accumulated case reports of vancomycin-related nephrotoxicity found that many of the patients had also received other known nephrotoxins, particularly aminoglycosides. Most of the rest had other confounding factors, or insufficient data regarding the possibility of such, that prohibited the clear association of vancomycin with the observed renal dysfunction. In 1994, in the largest systematic review to date, Cantu et al found that the use of vancomycin monotherapy was clearly documented in only three of 82 available cases in the literature. Prospective and retrospective studies attempting to evaluate the incidence of vancomycin-related nephrotoxicity have largely been methodologically flawed and have produced variable results. The most methodologically sound investigations indicate that the actual incidence of vancomycin-induced nephrotoxicity is around 5% to 7%. To put this into context, similar rates of renal dysfunction have been reported for cefamandole and penicillin, two reputedly non-nephrotoxic antibiotics. Additionally, evidence to relate nephrotoxicity to vancomycin serum levels is inconsistent. Some studies have indicated an increased rate of nephrotoxicity when trough levels exceed 10 mcg/ml, but others have not reproduced these results. Nephrotoxicity has also been observed with concentrations within the ‘therapeutic” range as well. Essentially, the reputation of vancomycin as a nephrotoxin is over-stated, and it has not been demonstrated that maintaining vancomycin serum levels within certain ranges will prevent its nephrotoxic effects, when they do occur.
Read more at Wikipedia.org