Barrier Therapeutics, Inc. (NASDAQ: BTRX), a
pharmaceutical company developing and commercializing products in the field
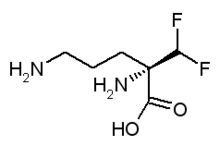
of dermatology, today announced the commercial launch of VANIQA®
(eflornithine hydrochloride) Cream, 13.9% in Canada. Barrier Therapeutics
acquired the exclusive right to distribute VANIQA® in Canada from Shire
Pharmaceutical Contracts Limited in June 2005. VANIQA® is the only
topical prescription product approved by Health Canada for slowing the
growth of unwanted facial hair in women.
"We are very excited about the launch of VANIQA® in Canada," said Joan
Chypyha, General Manager of Barrier Therapeutics in Canada. "Now,
physicians in Canada have available a unique treatment option for female
patients that suffer from excessive facial hair. Currently, this is the
only topical prescription product available in Canada for treating this
condition."
Excessive facial hair can be very distressing for women and can be
associated with social and psychological difficulties including anxiety and
social avoidance. VANIQA®, when used together with traditional hair
removal methods, reduces the growth rate of facial hair in women by
blocking an enzyme that is necessary for hair growth. VANIQA® has been
studied in over 1,800 women in clinical trials in which subjects were
treated twice daily for 24 weeks. Statistically significant improvements
were noted in as few as 4 weeks of treatment and at 24 weeks of treatment
VANIQA® was statistically and clinically superior to vehicle (p=0.001).
With its unique non-hormonal mode of action, it is effective on all hair
types and may offer both functional and emotional benefits to patients.
"The launch of VANIQA® in Canada represents a significant milestone for
Barrier Therapeutics as we continue to build upon our global
commercialization plans," said Al Altomari, Chief Commercial Officer.
"With an experienced sales and marketing team in place, we believe we are
well positioned to successfully market this product."
VANIQA® is now the second product marketed and sold in Canada by Barrier
Therapeutics. Solagé®(mequinol 2%, tretinoin 0.01%) Topical Solution,
for the treatment for age spots, is currently marketed and sold by Barrier
in both the United States and Canada.
To obtain more information about VANIQA®, call 1-866-440-5507.
About Barrier Therapeutics, Inc.
Barrier Therapeutics, Inc. is a pharmaceutical company focused on the
discovery, development and commercialization of pharmaceutical products in
the field of dermatology. The Company currently markets Solagé®
(mequinol 2%, tretinoin 0.01%) Topical Solution in the U.S. and Canada for
the treatment of solar lentigines, a common condition also known as "age
spots," and markets VANIQA® (eflornithine hydrochloride) Cream 13.9% for
slowing the growth of unwanted facial hair in women in Canada. Barrier has
eight product candidates in various stages of clinical development. The
four most advanced product candidates include Vusion(TM) (formerly known
as Zimycan(TM)) for the treatment of diaper dermatitis complicated by
candidiasis, which is under FDA review, and three products, which are in or
entering Phase 3 clinical trials for the treatment of seborrheic
dermatitis, onychomycosis, and congenital ichthyosis. Barrier has product
candidates in earlier stages of clinical development for the treatment of
acne, psoriasis and fungal infections. The Company is headquartered in
Princeton, New Jersey and has wholly owned subsidiaries in Geel, Belgium
and Ontario, Canada. Web site: http://www.barriertherapeutics.com.
Safe Harbor Statement
In addition to historical facts or statements of current condition, this
press release contains forward-looking statements within the meaning of the
"Safe Harbor" provisions of The Private Securities Litigation Reform Act of
1995, including statements regarding Barrier's plans for commencing
commercialization of VANIQA®. Forward-looking statements provide
Barrier's current expectations or forecasts of future events. Barrier's
performance and financial results could differ materially from those
reflected in these forward-looking statements due to the decisions of
regulatory authorities, Barrier's ability to implement its commercial
plans, the market acceptance of VANIQA®, general financial, economic,
regulatory and political conditions affecting the biotechnology and
pharmaceutical industries generally. For a discussion of these and other
risks and uncertainties that may effect the forward-looking statements
please see the risk factors in our Annual Report on Form 10K, which is on
file with the Securities and Exchange Commission. Given these risks and
uncertainties, any or all of these forward-looking statements may prove to
be incorrect. Barrier undertakes no obligation to update publicly any
forward-looking statement.
Contact:
Barrier Therapeutics, Inc.
Anne M. VanLent
609-945-1202
Barrier Therapeutics Canada
Joan Chypyha
905-773-0066
Noonan Russo
Jane Petrino
212-845-4274