Ventricular septal defects do not typically result in a continuous murmur. The only previously reported case involved a congenital ventricular septal defect. We report a ease of an acquired ventricular septal defect following a large anterior myocardial infarction resulting in a continuous murmur. The continous nature was confirmed both with 2D echocardiography and a specially equipped stethoscope in conjuntion with a handheld computer to provide a visual display of the murmur. A proposed mechanism for the continuous murmur was discussed.
Key words: cardiology physical diagnosis; coronary artery disease; echocardiography; myocardial infarction; shock
Abbreviations: LAD = left anterior descending; VSD = ventricular septal defect
**********
A 64-year-old man without a previously known history of coronary artery disease or cardiomyopathy was transferred from an outside facility after presenting with 36 h of continuous chest pain. His initial creatinine kinase level was 2,982 U/L with a troponin-I level of > 50 ng/mL. On hospital admission, the patient had ongoing chest pain with ST elevation in the anterior precordial leads. The patient was sent for cardiac catheterization; however, prior to this procedure, he developed third-degree heart block. The patient was resuscitated and a transvenous pacemaker was inserted. The diagnostic catheterization showed a proximal left anterior descending (LAD) artery occlusion. Percutaneous transluminal coronary angioplasty was attempted on the occluded LAD. Unfortunately, while attempting to cross the lesion there was a wire perforation of the LAD artery, and the procedure was aborted.
An intraaortic balloon pump was placed. Left ventricular pressures were 105/28 prior to insertion of this device. An echocardiogram was performed that showed a large hypokinetic segment involving the anterior, anteroseptal, and apical portions of the left ventricle with an estimated ejection fraction of 15%. There was no evidence for a pericardial effusion or tamponade. A repeat echoeardiogram was performed the following day, and the findings were unchanged. The patient remained hemodynamically stable and was started on therapy with aspirin, clopidogrel, atorvastatin, metoprolol tartrate, and ramipril. Treatment with the intraaortic balloon pump was discontinued after 3 days.
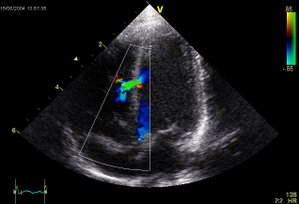
On the fifth hospital day, a harsh continuous III/VI murmur along the lower left sternal border was noted during physical examination. The murmur intensified during systole but continued throughout diastole. A waveform and spectral display of the murmur are shown in Figure 1. The murmur was recorded using a standard stethoscope equipped with an electronic recording device (Prosteth; Point of Care., Toronto, CA) and was displayed on a standard handheld computer (IPAC; Hewlett-Packard; Palo Alto, CA) possessing software designed for this purpose (Sounds Easy; Point of Care). A repeat echocardiogram then showed a small muscular ventricular septal defect (VSD) near the apex but was otherwise unchanged (Fig 2). The color Doppler image displayed left-to-right shunting both during systole and diastole. To our knowledge, this is the first continuous murmur associated with an acquired VSD. In fact, there has been only one instance of a continuous VSD murmur reported in a patient with a congenital VSD. (1,2) We postulate that the murmur was continuous as a result of elevated left ventricular diastolic pressure secondary to the acute anterior myocardial infarction. A marked pressure disparity between the left and right ventricles during diastole could explain the continued left-to-right shunting during this latter period. Continued shunting during diastole was likely facilitated by the small diameter of the defect, which prevented the rapid equilibration of pressures between the two chambers.
[FIGURES 1-2 OMITTED]
The specially equipped stethoscope in conjunction with a handheld computer used in this study was valuable in the assessment and teaching of cardiac sounds. In this instance, the visual displays provided confirmation of the continuous nature of this murmur; moreover, this apparatus allowed for repeated playback at both full speed and half-speed, providing a means for additional auditory review.
REFERENCES
(1) Assey ME, Stalheim RM, Usher BW. Ventricular septal defect with aneurysm of the membranous septum presenting as a systolic and early diastolic murmur. Chest 1979; 75:504-506
(2) Tavel ME. Clinical phonocardiography and external pulse recording. 4th ed. Chicago, IL: Year Book Medical Publishers, 1985; 181 and 326
Manuscript received January 30, 2005; revision accepted March 23, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to: Jeff Olson, St Vincent's Hospital, Cardiology, 8333 Naab Rd, Indianapolis, IN 46260; e-mail: jakjiolson@ sbcglobal.net
* From the Department of Cardiology (Dr. Olson), St Vincent's Hospital, Indianapolis, IN; and the Cardiology Department (Dr. Tavel), The Care Group, Indianapolis, IN.
COPYRIGHT 2005 American College of Chest Physicians
COPYRIGHT 2005 Gale Group