Aortopulmonary window is a rare cardiac anomaly that results from incomplete division of the aortopulmonary septum between the ascending aorta and the main pulmonary artery. Noninvasive diagnosis of this lesion is sometimes difficult because of associated cardiac anomalies.
Although the two-dimensional echocardiographic features of aortopulmonary window are well-established,[1-3] Doppler color flow imaging of this anomaly has not been reported sufficiently.[4,5] We report the case of an infant who had an aortopulmonary window associated with a ventricular septal defect, in whom Doppler color flow imaging demonstrated blood flow through an echo dropout between the ascending aorta and the main pulmonary trunk.
Case Report
A two-year-old girl was admitted to the hospital for evaluation of a cardiac murmur. She was born at full term, and a systolic murmur was discovered when she was one month old. Digoxin and diuretics were then administered because of congestive heart failure.
At the age of two years, she was admitted to the hospital because of respiratory infection and congestive heart failure. Physical examination revealed a grade 3/6 pansystolic murmur along the left sternal border and a grade 2/6 diastolic murmur at the cardiac apex. Her liver was enlarged. At this time, the patient was referred to our hospital because of continuing congestive heart failure.
The chest roentgenogram showed cardiomegaly, with a cardiothoracic ratio of 0.58 and increased pulmonary vascularity. The electrocardiogram showed left axis deviation of the mean QRS axis as well as biatrial and biventricular hypertropy. Two-dimensional and Doppler echocardiography (A Toshiba model SSH-65A system with a 3.75 MHz transducer; Toshiba, Tokyo, Japan) showed a large ventricular septal defect, a dilated ascending aorta, and a dilated main pulmonary trunk with normally positioned bilateral pulmonary arteries.
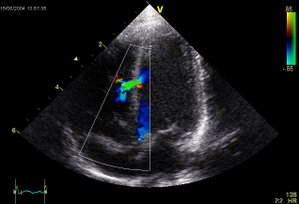
Suprasternal, long-axis scanning of the aortic arch demonstrated a large communication (12mm) between the aortic arch and the main pulmonary trunk (Fig 1, top). Color flow imaging revealed a flow signal crossing the communication from the aorta to the pulmonary trunk during systole (Fig 1, bottom). High parasternal, short-axis scanning above the aortic and pulmonary valves confirmed the diagnosis. Diastolic flow reversal was observed proximal to the aortic isthmus by pulsed Doppler echocardiography, which indicated that the aortic runoff was located proximal to where the ductus arteriosus opened (Fig2).
Cardiac catheterization was performed to obtain hemodynamic information. There was a marked elevation of pulmonary arterial pressure (104/56 mm Hg; mean 77 mm Hg), with a large pulmonary flor (Qp/Qs=2.98) and an elevated pulmonary resistance ration (Rp/Rs=0.5). Right heart catheterization revealed left-to-right shunting at the ventricular and arterial levels. Aortography opacified the ascending aorta, the main pulmonary trunk, and the pulmonary arteries simultaneously, confirming the diagnosis of aortopulmonary window and ventricular septal defect.
Operation was undertaken with cardiopulmonary bypass, supplemented by circulatory arrest. The aorticopulmonary septal defect (10 x 12 mm) was closed with a prosthetic patch by the transpulmonary approach. The ventricular septal defect (20 x 10 mm) was closed with the same material via the transatrial approach. Postoperative pulmonary arterial pressure was 47/17 mm Hg (mean, 27 mm Hg) and the postoperative course was uneventful.
Discussion
The present patient had an aortopulmonary window with a ventricular septal defect and the diagnosis was confirmed by echocardiography: Accurate diagnosis of aortopulmonary window is extremely difficult on clinical grounds when it coexists with a ventricular septal defect. The heart murmur of this anomaly is often mistaken for the murmur of a high ventricular septal defect, because although a continuous murmur may be present in patients with aortopulmonary window, more often there is only a systolic murmur that is generally heard along the upper left sternal border. In this case, there was no continuous murmur because there was little left-to-right diastolic flow through the aortopulmonary window, due both to the elevated pulmonay arterial diastolic pressure and to the large size of the communication.
The other cardiac anomaly that can mimic the findings in this case is a large ventricular septal defect associated with patent ductus arteriosus. Aortopulmonary window can be distinguished from patent ductus arteriosus by the detection of diastolic flow reversal proximal to the aortic isthmus using pulsed Doppler echocardiography.[6]
In recent years, two-dimensional echocardiography has been found to be extremely helpful in the noninvasive diagnosis of various conotruncal anomalies. In many normal individuals (especially infants), the contiguous proximal aortic and pulmonary arterial walls are thin and many appear as a false dropout on two-dimensional echocardiography. Therefore, this could potentially result in a false diagnosis of aortopulmonary window. However, Droppler color flow imaging can verify the presence of a defect by displaying evidence of flow across the echo-free space into the main pulmonary artery from the aorta.[4.5] In this case, two-dimensional echocardiography showed a large communication between the aortic arch and the main pulmonary trunk, and Droppler color flow imaging clearly indicated the flow between the aorta and the pulmonary artery. A large ventricular septal defect was also confirmed by both two-dimensional echocardiography and Doppler color flow imaging. Thus, the combination of two-dimensional echocardiography and Doppler color flow imaging as well as pulsed Doppler echocardiography can rule out other diseases that mimic or are associated with aortopulmonary window.
References
[1.] King DH, Huhta J, Gutgesell HP, Ott DA. Two-dimensional
echocardiographic diagnosis of anomalous origin of the pulmonary
artery from the aorta; differentiation from aortopulmonary window.
J Am Coll Cardiol 1984; 4:351-55 [2.] Satomi G, Nakamura K, Imai Y, Takao A. Two-dimensional;
echocardiographic diagnosis of aortopulmonary window. Br Heart
J 1980;43:351-56 [3.] Mendoza DA, Tadashi V, Nishioka K, Yokota T, Mikawa H,
Nomoto S, et al. Aortopulmonary window, aortic origin of the
right pulmonary artery, and interrupted aortic arch: detection
by two-dimensional and color Doppler echocardiography in an
infant. Pediatr Cardiol 1988; 7:49-52 [4.] Rice MJ, Seward JB, Hagler DJ, Mair DD, Tajik AJ. Visualization
of aortopulmonary window by two-dimensional echocardiography.
Mayo Clin Proc 1982; 57:482-87 [5.] Alboliras ET, Chin AJ, Barber G, Helton JG, Pigott JD. Detection
of aortopulmonary window by pulsed and color Doppler echocardiography.
Am Heart J 1988; 115:900-02 [6.] Daniels O, Hopman J, Deknecht A, Vanoort A, Busch H. Pulsed
Doppler echocardiography in patients with aorto-pulmonary
connection. Acta Paediatr Scand 1986; 329(suppl):44-52
COPYRIGHT 1992 American College of Chest Physicians
COPYRIGHT 2004 Gale Group