Vitamin B12 Deficiency
The term vitamin B12 (or B12 for short) is used in two different ways. In a broader sense it refers to a group of Co-containing compounds known as cobalamins - cyanocobalamin (an artefact formed as a result of the use of cyanide in the purification procedures), hydroxocobalamin and the two coenzyme forms of B12, methylcobalamin (MeB12) and 5-deoxyadenosylcobalamin (adenosylcobalamin - AdoB12). more...
In a more specific way, the term B12 is used to refer to only one of these forms, cyanocobalamin, which is the principal B12 form used for foods and in nutritional supplements.
Pseudo-B12 refers to B12-like substances which are found in certain organisms, such as Spirulina spp. (blue-green algae, cyanobacteria). However, these substances do not have B12 biological activity for humans.
Structure
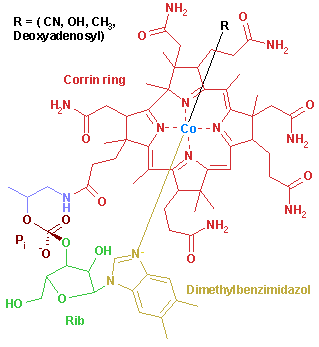
B12 is the most chemically complex of all the vitamins. B12's structure is based on a corrin ring, which, although similar to the porphyrin ring found in haem, chlorophyll, and cytochrome, has two of the pyrrole rings directly bonded. The central metal ion is Co (cobalt). Four of the six coordinations are provided by the corrin ring nitrogens, and a fifth by a dimethylbenzimidazole group. The sixth coordination partner varies, being a cyano group (-CN), a hydroxyl group (-OH), a methyl group (-CH₃) or a 5'-deoxyadenosyl group (here the C5' atom of the deoxyribose forms the covalent bond with Co), respectively, to yield the four B12 forms mentioned above. The covalent C-Co bond is the only carbon-metal bond known in biology. (p.32)
Synthesis
B12 cannot be made by plants or by animals, as the only type of organism that have the enzymes required for the synthesis of B12 are bacteria (eubacteria, archaebacteria).
Functions
Coenzyme B12's reactive C-Co bond participates in two types of enzyme-catalyzed reactions: (p.675)
- Rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcochol, or an amine.
- Methyl (-CH₃) group transfers between two molecules.
In humans there are only two coenzyme B12-dependent enzymes:
- MUT which uses the AdoB12 form and reaction type 1 to catalyze a carbon skeleton rearrangement (the X group is -COSCoA). MUT's reaction converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats (for more see MUT's reaction mechanism)
- MTR, a methyl transfer enzyme, which uses the MeB12 and reaction type 2 to catalyzes the conversion of the amino acid Hcy into Met (for more see MTR's reaction mechanism).
Read more at Wikipedia.org