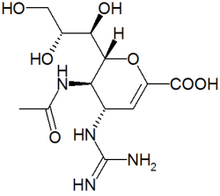
Zanamivir represents a new class of antiviral agents known as neuraminidase inhibitors. In vitro and in vivo studies have demonstrated that zanamivir exerts activity against influenza virus types A and B. Monto and colleagues evaluated the clinical efficacy and safety of zanamivir in a double-blind randomized, placebo-controlled trial.
The multicenter study included 1,256 patients who were at least 13 years of age (mean age: 34, 35 and 36 years in each treatment group) and had symptoms of influenza for 48 hours or less. Symptoms of influenza included fever plus at least two of the following symptoms: cough, sore throat, myalgias and headache. Influenza was confirmed by laboratory tests in 722 (57.5 percent) of the patients. The study included 158 high-risk patients, such as those over the age of 65 and those with chronic cardiovascular, respiratory, metabolic or endocrine disease.
Patients were randomized into four treatment groups: 10 mg of zanamivir by oral inhalation and 6.4 mg by nasal spray, administered twice daily (419 patients); 10 mg of zanamivir by oral inhalation and 6.4 mg by nasal spray, administered four times daily (415 patients); twice-daily administration of placebo by both routes, and four-times-daily administration of placebo by both routes. The placebo groups included 422 patients. Patients took the medication for five days. The first dose was given at the initial clinic visit, when patients received instructions on administering their therapy. Study participants were also given dextromethorphan and acetaminophen but were told to use these drugs only if it was absolutely necessary.
Patients kept a record of oral temperature and severity of symptoms for 10 days. Symptoms were rated on a scale of zero to 5, with a score of 5 corresponding to the severest symptoms. They also noted any sleep disturbances, the level of their ability to perform daily functions and their overall health status.
Analysis of the data revealed that zanamivir reduced by one day the median number of days that clinically significant symptoms were present. Overall, symptoms were present for six days in the patients receiving zanamivir compared with seven days in those receiving placebo. The duration of illness was decreased by 1.0 to 1.5 days in patients who began taking zanamivir within 30 hours of the onset of symptoms. Symptoms were alleviated in a median of 5.5 days among the patients who began receiving twice-daily zanamivir within 30 hours of the onset of symptoms. In the four-times-daily group, symptoms subsided in a median of 5.0 days. In the placebo group, symptoms subsided in 6.5 days. No statistically significant difference in duration of illness was found between the two zanamivir-treatment groups. Among the high- risk patients with laboratory-confirmed influenza, zanamivir reduced the duration of symptoms by 2.75 days in the twice-daily treatment group and by 3 days in the four-times-daily treatment group.
The most common adverse events were nasal symptoms, headaches, bronchitis and gastrointestinal upset. The incidence of these adverse events ranged from 1 to 4 percent in the zanamivir treatment groups. A comparable incidence of adverse events was observed in the placebo group.
The authors conclude that zanamivir is a safe and effective treatment for influenza virus types A and B. The benefits seem to be greatest when the illness is most severe and when antiviral therapy is initiated early in the course in high-risk patients. Thus far, viral resistance to the drug has not been observed.
Monto AS, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J Infect Dis August 1999;180:254-61.
editor's note: Zanamivir (Relenza) was labeled for the treatment of influenza without complications by the U.S. Food and Drug Administration in July 1999. Additional data and clinical experience will further elucidate the effectiveness of this agent in the treatment of influenza.
COPYRIGHT 2000 American Academy of Family Physicians
COPYRIGHT 2000 Gale Group