For 3 decades, scientists have been experimenting with medications that stifle the influenza virus. Two drugs, amantadine and rimantadine, can impede the disease and limit symptoms. However, neither works against one of the two major strains of influenza, and both can be eluded by the evolving virus. In addition, amantadine causes some disagreeable side effects, including confusion and nightmares.
A new antiviral--zanamivir--seems to ease or eliminate many symptoms of either flu strain if taken within a day or two of the disease's onset, and the viruses seem powerless to develop resistance to it. Now, researchers find that the medication also works as a preventive against both strains of influenza when taken for 4 weeks during the height of flu season. In the July 7 JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION, they also report that zanamivir shows no side effects.
The two classes of disease-causing influenza virus are dubbed A and B. "The potential of having access to a new class of antiviral agents that can be used in the prevention of both influenza A and B is very exciting," says Linda C. Lambert of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
To test the drug, researchers gave each of 1,107 people in Michigan and Missouri an inhaler that dispensed a powder and instructed them to take one dose per day. Half of the people received an inert substance, or placebo; the rest got zanamivir. Neither researchers nor participants knew which was the placebo.
Of 554 people getting the placebo, 34 subsequently came down with the fin, and 19 of them also ran a fever. Of 553 getting zanamivir, only 11 got the flu and just 3 had a fever.
About 14 percent of each group had already received a flu shot. Among these people, zanamivir imparted additional protection and didn't hinder the vaccine's effectiveness, says study coauthor Arnold S. Monto, of the University of Michigan in Ann Arbor. Zanamivir is made by Glaxo Wellcome of Durham, N.C.
"This is a noteworthy piece of science," says Fred Y. Aoki, of the University of Manitoba in Winnipeg. "It confirms ... that drugs for prevention of the flu are an important adjunct to vaccine."
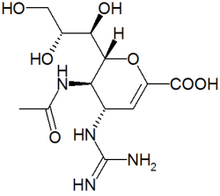
Flu virus consists primarily of RNA molecules wrapped in proteins. Once a virus has invaded a cell and multiplied, new virus particles emerge from the cell bound together. One viral protein, an enzyme called neuraminidase, is required for the bundle to unglue itself so that individual virus particles can infect other cells.
Zanamivir foils the flu by binding to neuraminidase and deactivating it. The virus particles then stay bundled. The molecular docking site the drug uses to attach to neuraminidase doesn't vary much among different strains of flu virus, so scientists call it a conserved site.
In earlier laboratory efforts intended to induce the virus to become resistant, it didn't find a way to substitute an enzyme impervious to the drug, Aoki says.
This gives zanamivir an advantage over the flu vaccine. The immunization, which uses a disabled virus to elicit an antibody response to the active flu virus, aims at a moving target. The vaccine-triggered antibodies recognize the flu virus less often as it spreads in a population, mutating rapidly. Even in young adults, a flu shot is at best 70 to 90 percent effective. Indeed, the vaccine used in the 1997-1998 flu season, when the scientists conducted the new study, proved only marginally effective.
Many scientists believe it's only a matter of time before doctors will be up against a highly lethal flu, such as the 1918 strain that killed tens of millions of people. In such a pandemic, zanamivir may work against the virus even as flu shots fail because it will still be able to dock to neuraminidase's conserved Site, Monto says.
"There's no doubt this has the potential to become a really important stopgap measure in case of a new pandemic," Aoki says. "The limiting factor is in the logistics of having enough drugs readily available to distribute in such a circumstance."
The drug may initially prove most valuable to people who are allergic to flu vaccine or those whose immune system has been compromised by anticancer medications or drugs that limit rejection after an organ transplant, says W. Paul Glezen, of Baylor College of Medicine in Houston. Among elderly people, whose immune response is weakened, the vaccine is only 30 to 50 percent effective.
Researchers still need to test zanamivir on high-risk patients, elderly people, and those with asthma, he says. The participants in Monto's study averaged 29 years old. "These were [mostly] healthy young people," Glezen notes.
Zanamivir might also be useful as an interim measure among people who are exposed to the virus before getting a flu shot. Daily doses may fend off the disease while the vaccine mounts its antibody response, Glezen says.
COPYRIGHT 1999 Science Service, Inc.
COPYRIGHT 2000 Gale Group