Most family physicians are comfortable prescribing antidepressants, but antipsychotic medications are less commonly prescribed and therefore less familiar. Antipsychotic drugs effectively treat psychosis caused by a variety of conditions (Table 1). Psychotic symptoms are classified as either positive or negative. Positive symptoms include hallucinations, delusions, thought disorders (manifested by marked incoherence, derailment, tangentiality), and bizarre or disorganized behavior. Negative symptoms include anhedonia, flattened affect, apathy, and social withdrawal. (1)
Psychotic symptoms in elderly patients always should be investigated thoroughly, and underlying medical conditions should be identified and treated. Although a family physician is less likely to manage schizophrenia in elderly patients, it is quite common for family physicians to treat patients who have Alzheimer's disease and Parkinson's disease. These patients frequently have psychotic symptoms that are treated without a specialist's aid.
Typical antipsychotic drugs, such as haloperidol (Haldol), traditionally have been used to control psychotic and behavior disturbances in elderly patients, but these drugs have troubling side effects. Extrapyramidal symptoms can cause stiffness, immobility, and falls and are associated with significant morbidity. The newer atypical antipsychotic drugs offer distinct advantages over older agents, including decreased extrapyramidal symptoms and improved efficacy in treatment of the negative symptoms of psychosis. Family physicians should become familiar with the use of atypical antipsychotic drugs in elderly patients (Table 2).
Atypical antipsychotic drugs are especially useful in treating symptoms associated with common neuropsychiatric disorders, such as Alzheimer's disease and Parkinson's disease. (2-4) As the number of elderly people in the United States increases, the use of atypical antipsychotic drugs is expected to increase substantially. The National Institutes of Health estimates that there will be 8.5 million Americans with Alzheimer's disease by the year 2030. (5) Psychotic symptoms are present in at least 25 percent of mildly demented patients with Alzheimer's disease and in 50 percent of patients with advanced Alzheimer's disease. (6) Among persons older than 65 years, the incidence of Parkinson's disease is 2 percent. (7) Hallucinations occur in up to 20 percent of patients with Parkinson's disease; delusions, paranoia, and subcortical dementia also may occur. (8,9)
Treatment of Behavior Disturbances
Initial interventions for behavior disturbances should include cognitive, environmental, and social techniques. Many demented patients with behavior disturbances will not need psychotropic medication but can be managed successfully with nonpharmacologic techniques, such as the use of familiar objects, maintenance of sleep-wake cycles, redirection, and frequent reorienting (verbally or by posting a calendar in their room).
There are many differences of opinion about when medications are indicated. There is even conflicting evidence about the efficacy of medications in treating behavior symptoms in dementia. (10,11) Therefore, decisions to use these medications should be made on a case-by-case basis. Most guidelines call for the use of medications only when other methods have failed. The Health Care Financing Administration has produced regulations governing the use of psychotropic medications in nursing homes. Several authors have adapted these regulations into clinically useful guidelines (Table 3). (12,13)
Typical Antipsychotic Agents
Psychotic symptoms traditionally have been treated with so-called "typical" antipsychotic drugs--older agents such as haloperidol and thioridazine (Mellaril). These medications have a variety of pharmacologic actions. Their ability to block the dopamine ([D.sub.2]) receptor in the mesolimbic system reduces positive symptoms of psychosis. The [D.sub.2] blockade in the nigrostriatal pathway causes extrapyramidal symptoms, which include drug-induced parkinsonism, akathisia, acute dystonia, and tardive dyskinesia. The [D.sub.2]-receptor blockade in the tuberoinfundibular pathway increases serum levels of prolactin, which may present clinically as breast tenderness, galactorrhea, or erectile dysfunction. (1) Younger patients may present with amenorrhea.
Atypical Antipsychotic Agents
The pharmacodynamic action of atypical antipsychotic drugs is attributed to their action on both the serotonergic and dopaminergic systems. Some experts argue that this combination of relative effects on dopamine and serotonin allows atypical antipsychotic drugs to treat both positive and negative symptoms of psychosis while producing fewer extrapyramidal symptoms and decreasing iatrogenic hyperprolactinemia. (14)
There is growing concern over recent reports of hyperglycemia in patients who are taking certain atypical antipsychotic drugs. The increased rate of hyperglycemia appears to be independent of weight gain. These findings have led some investigators to recommend screening for diabetes twice a year in patients who are taking atypical antipsychotic drugs. (15)
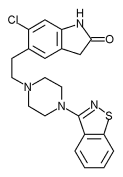
RISPERIDONE
Risperidone (Risperdal) usage in Alzheimer's disease and Parkinson's disease has mixed results. Significant evidence demonstrates the efficacy of risperidone in the treatment of psychotic and behavior symptoms in patients with dementia. (10,16,17) [References 10 and 17--Evidence level A, randomized controlled trials (RCTs)] However, risperidone exacerbates movement disorders in patients with Parkinson's disease and has been shown to be less effective than clozapine (Clozaril) in controlling psychosis in these patients. (18-20)
Initial dosages of 0.25 mg per day are titrated slowly upward to achieve the desired effect. In two studies (10,17) documenting the efficacy of risperidone in patients with dementia, the mean dosages were 1.1 mg per day and 1.2 mg per day. Risperidone causes extrapyramidal symptoms in a dosage-dependent manner, so the lowest effective dosage is used.
Significant side effects of risperidone include insomnia, hypotension, weight gain, and extrapyramidal symptoms. Extrapyramidal symptoms are more likely when the dosage is more than 6 mg per day. (21) Risperidone is metabolized by the cytochrome P450 2D6 system. Any medication that affects this enzyme (e.g., celecoxib [Celebrex], amiodarone [Cordarone], cimetidine [Tagamet], fluoxetine [Prozac], paroxetine [Paxil]) can alter the efficacy of risperidone. Risperidone causes a significant elevation in prolactin levels. Caution should be used when prescribing risperidone with other medications that cause hypotension.
OLANZAPINE
Studies indicate that olanzapine (Zyprexa) is an effective treatment for psychotic and behavior symptoms in patients with Alzheimer's disease. (22,23) [Reference 22--Evidence level A, RCT] However, in patients with Parkinson's disease, olanzapine was found to increase motor symptoms and to be less effective than clozapine. Therefore, current recommendations discourage the use of olanzapine in patients with Parkinson's disease. (24) [Evidence level B, uncontrolled study]
In patients with Alzheimer's disease and psychotic symptoms, dosages should start at 1.25 to 2.5 mg per day and increase to 5 mg per day, if necessary. Surprisingly, dosages of 10 or 15 mg per day are less effective than dosages of 5 mg per day. (22-26) Common side effects of olanzapine include sedation and weight gain. Special considerations in elderly patients include the risk of orthostatic hypotension and seizures. In pre-marketing testing, olanzapine was associated with a 0.9 percent rate of seizures. Seizures occurred in patients with confounding factors; consequently, this medication should be used with caution in patients who have a lowered seizure threshold. (21) Olanzapine is metabolized by the cytochrome P450 1A2 system, as well as multiple other hepatic pathways, and therefore has a low potential for drug-drug interactions.
QUETIAPINE
Quetiapine (Seroquel) has shown promise in the treatment of psychosis in elderly patients with Alzheimer's disease and Parkinson's disease. It improves psychosis in patients with Parkinson's disease without exacerbating movement disorders. This feature has led some experts to recommend it as the first-line agent for treatment of psychosis in patients with Parkinson's disease. (27,28) [Reference 28--Evidence level B, uncontrolled study] It has been shown to be safe in patients with Alzheimer's disease, but more controlled trials are needed before its use in these patients can be endorsed. (29)
Quetiapine should be initiated at a dosage of 12.5 mg at bedtime and titrated every three to five days until the desired effect is achieved or side effects emerge. Common side effects include sedation, headache, and orthostatic hypotension. Cataract formation was noticed in pre-marketing studies, but a causal relationship has not been found. Screening for cataract formation is recommended at the initiation of therapy and at six-month intervals thereafter. (21) Quetiapine is metabolized by the cytochrome P450 3A4 system. Serum levels of quetiapine can be affected by inducers or inhibitors of this enzyme system (e.g., ketoconazole [Nizoral], erythromycin, diltiazem [Cardizem], fluoxetine, ciprofloxacin [Cipro], grapefruit juice, and phenytoin [Dilantin]). (21)
ZIPRASIDONE
Because ziprasidone (Geodon) was recently released, clinical data are lacking to support its use in patients with either Parkinson's disease or Alzheimer's disease. Side effects of ziprasidone include rash, hypertension, and (rarely) non-dose-dependent QT-interval prolongation. Ziprasidone should be avoided in patients at risk for significant electrolyte abnormalities and in patients with histories of significant cardiovascular illness, recent acute myocardial infarction, uncompensated heart failure, and cardiac arrhythmia. Ziprasidone is metabolized by the cytochrome P450 3A4 system. (21)
CLOZAPINE
Research on clozapine in the geriatric population has had mixed results. Clozapine is highly effective in treating psychosis in patients with Parkinson's disease. (30) [Evidence Level A, RCT] The American Academy of Neurology states that clozapine appears to be the most effective agent in the treatment of drug-induced psychosis in patients with Parkinson's disease. (18,30) Clozapine has shown some efficacy in controlling psychosis and behavior disturbances in patients with Alzheimer's disease. (16,31) Initial dosages can start as low as 6.5 mg per day and are titrated upward.
Clozapine is well known for its side effects, which include agranulocytosis (with a fatality rate as high as 30 percent), sedation, seizures, sialorrhea, hypotension, weight gain, tachycardia, and hyperthermia. (21) A complete blood count must be checked frequently in patients taking this medication. Because of its serious and potentially lethal side effects, clozapine generally is used only after other options have failed. Clozapine is metabolized by the cytochrome P450 1A2 and 2D6 systems.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Air Force Medical Corps or the U.S. Air Force at large.
The authors report that they do not have any conflicts of interest. Sources of funding: none reported.
REFERENCES
(1.) Hales RE, Yudofsky SC, Talbott JA. The American Psychiatric Press Textbook of psychiatry. 3d ed. Washington, D.C.: American Psychiatric Press, 1999.
(2.) Doody RS, Stevens JC, Beck C, Dubinsky RM, Kaye JA, Gwyther L, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1154-66.
(3.) Wolters EC. Dopaminomimetic psychosis in Parkinson's disease patients: diagnosis and treatment. Neurology 1999;52(suppl 3):s10-3.
(4.) Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA 1997;278:1363-71.
(5.) National Institute on Aging, Alzheimer's Disease Education & Referral Center (National Institute on Aging), National Institutes of Health. Progress report on Alzheimer's disease 1999. Bethesda, Md.: National Institutes of Health, National Institute on Aging, 1999.
(6.) Cummings JL, Miller B, Hill MA, Neshkes R. Neuropsychiatric aspects of multi-infarct dementia and dementia of the Alzheimer type. Arch Neurol 1987;44:389-93.
(7.) Aminoff MJ. Parkinson's disease. Neurol Clin 2001;19:119-28,vi.
(8.) Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol 1996;53:1265-8.
(9.) Fogel BS, Schiffer RB, Rao SM, eds. Neuropsychiatry. Baltimore: Williams & Wilkins, 1996:807-9.
(10.) De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, et al. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999;53:946-55.
(11.) Teri L, Logsdon RG, Peskind E, Raskind M, Weiner MF, Tractenberg RE, et al. Treatment of agitation in AD: a randomized, placebo-controlled clinical trial. Neurology 2000;55:1271-8.
(12.) Ruby CM, Kennedy DH. Psychopharmacologic medication use in nursing home care: indicators for surveyor assessment of the performance of drug regimen reviews, recommendations for monitoring, and non-pharmacologic alternatives. Clin Fam Pract 2001;3:577-98.
(13.) Gurvich T, Cunningham JA. Appropriate use of psychotropic drugs in nursing homes. Am Fam Physician 2000;61:1437-46.
(14.) Stahl SM. Essential psychopharmacology: neuroscientific basis and practical application. 2d ed. New York, N.Y.: Cambridge University Press, 2000.
(15.) Luna B, Feinglos MN. Drug-induced hyperglycemia. JAMA 2001;286:1945-8.
(16.) Tariot PN, Ryan JM, Porsteinsson AP, Loy R, Schneider LS. Pharmacologic therapy for behavioral symptoms of Alzheimer's disease. Clin Geriatr Med 2001;17:359-76.
(17.) Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry 1999;60:107-15.
(18.) Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease (2001): treatment guidelines. Neurology 2001;56 (suppl 5):S1-88.
(19.) Mohr E, Mendis T, Hildebrand K, De Deyn PP. Risperidone in the treatment of dopamine-induced psychosis in Parkinson's disease: an open pilot trial. Mov Disord 2000;15:1230-7.
(20.) Ellis T, Cudkowicz ME, Sexton PM, Growdon JH. Clozapine and risperidone treatment of psychosis in Parkinson's disease. J Neuropsychiatry Clin Neurosci 2000;12:364-9.
(21.) Mosby's GenRx: a comprehensive reference for generic and brand prescription drugs. 11th ed. St. Louis, Mo.: Mosby, 2001.
(22.) Street J, Mitan S, Tamura R, et al. Olanzapine in the treatment of psychosis and behavioral disturbances associated with Alzheimer's disease. Eur J Neurology 1998;5:S39.
(23.) Satterlee WG, Reams SG, Burns PR, Hamilton S, Tran PV, Tollefson GD. A clinical update on olanzapine treatment in schizophrenia and in elderly Alzheimer's disease patients. Psychopharmacol Bull 1995;31:534.
(24.) Goetz CG, Blasucci LM, Leurgans S, Pappert EJ. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology 2000;55:789-94.
(25.) Jeste DV, Rockwell E, Harris MJ, Lohr JB, Lacro J. Conventional vs. newer antipsychotics in elderly patients. Am J Geriatr Psychiatry 1999;7:70-6.
(26.) Daniel DG. Antipsychotic treatment of psychosis and agitation in the elderly. J Clin Psychiatry 2000; 61(suppl 14):49-52.
(27.) Fernandez HH, Friedman JH, Jacques C, Rosenfeld M. Quetiapine for the treatment of drug-induced psychosis in Parkinson's disease. Mov Disord 1999; 14:484-7.
(28.) Dewey RB Jr, O'Suilleabhain PE. Treatment of drug-induced psychosis with quetiapine and clozapine in Parkinson's disease. Neurology 2000;55:1753-4.
(29.) Tariot PN, Salzman C, Yeung PP, Pultz J, Rak IW. Long-term use of quetiapine in elderly patients with psychotic disorders. Clin Ther 2000;22:1068-84.
(30.) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson's disease. The Parkinson Study Group. N Engl J Med 1999;340: 757-63.
(31.) Menza MA, Liberatore BL. Psychiatry in the geriatric neurology practice. Neurol Clin 1998;16:611-33.
CHARLES D. MOTSINGER, CAPT, USAF, MC, is a family practice psychiatrist and chief of the life-skills support center at Osan Air Force Base, South Korea. He received his medical degree from the Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine, Bethesda, Md., and completed a residency in the combined National Capital Consortium family practice/psychiatry program, also in Bethesda.
GREGORY A. PERRON, CAPT, USAF, MC, is a family physician and faculty member in the Malcolm Grow Medical Center family practice program at Andrews Air Force Base, Md. He received his medical degree from the Washington University School of Medicine, St. Louis, and completed a family practice residency at Malcolm Grow Medical Center.
TIMOTHY J. LACY, LTCOL, USAF, MC, is assistant professor of psychiatry and family practice at the Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine. He also is program director for the combined National Capital Consortium family practice/psychiatry residency program.
Address correspondence to Capt. Charles D. Motsinger, 715 West View Terr., Alexandria, VA 22301 (e-mail: charles.motsinger@osan.af.mil). Reprints are not available from the authors.
Richard W. Sloan, M.D., R.Ph., coordinator of this series, is chairman and residency program director of the Department of Family Medicine at York (Pa.) Hospital and clinical associate professor in family and community medicine at the Milton S. Hershey Medical Center, Pennsylvania State University, Hershey, Pa.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group