NEW YORK -- "If you suspect you or a loved one has suffered adverse effects resulting from Vioxx use ... recites one television commercial paid for by product liability litigators. The ads are becoming more and more prevalent--prime time on local television networks now that the first verdict of $253 million has bloodied the product liability waters. And that spells bad news for Merck.
Already facing some 5,000 individual lawsuits, the money verdict is expected to drive more people to sue Before the statute of limitations on Vioxx lawsuits expires this time next year. Some analysts have speculated as many as 100,000 cases may be filed claiming damages from the use of Vioxx.
Whether the withdrawal of a pharmaceutical product is self-imposed or initiated by the Food and Drug Administration, more and more of that action precipitates a flood of class-action and individual lawsuits seeking damages--damages that are payed out in the billions.
To date, Bayer has spent $1.1 billion settling more than 3,000 cases revolving around its statin drug Baycol, withdrawn in 2001. There are approximately 6,000 more cases worldwide, the company stated earlier this year. And Wyeth has set aside $21 billion so far in settlements of its weight-loss drug fen phen.
With some 5,000 Vioxx liability cases facing Merck, the pharmaceutical company's total payout may very well eclipse that of either Bayer or Wyeth--Merck's potential liability is pegged at a high of $50 billion (and a low of $4 billion). But right now, it's really too early to tell, analysts are saying.
"We do not think investors should apply [the Texas] ruling to the thousands of cases Merck faces," stated David Risinger in a recent Merrill Lynch analyst note. At the end of the day, that headline-grabbing settlement running in the hundreds of millions of dollars is going to be capped well under $30 million under Texas law, and the case may very well be appealed.
Rather, people should withhold judgment regarding Merck's potential liability until a few more verdicts are in--trials in New Jersey and again in Texas are expected to begin this month with a federal trial slated for later this year.
"Merck's win/loss rate in the next half-dozen or so trials is likely to drive investor sentiment regarding the company's theoretical liability," Risinger said.
It certainly doesn't look promising, however. According to published reports, Merck's defense was too clinical and sailed over the heads of jurors. In addition, jurors are zeroing in on the fact that Merck knew about the link to heart disease and Vioxx all along, citing internal communications that date well before the recall in 2004.
All of Merck's troubles have fueled merger speculation--analysts have been considering a marriage between Merck and Schering-Plough, its pharma partner on Zetia, for years. A more likely scenario would be a hostile takeover, especially if Merck's share value dips too low. However, many analysts have been skeptical regarding such a move--in addition to assuming the Vioxx liability, Merck is on the verge of losing two blockbuster medicines to generic competition and doesn't have the pipeline to restock those lost revenues in the coming years.
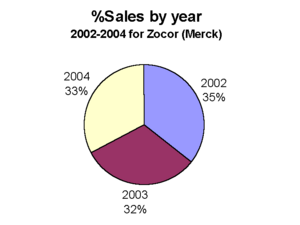
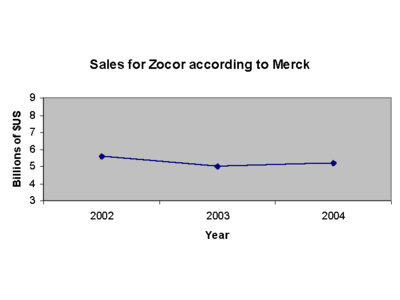
Indeed, Merck is looking at losing its biggest drug, Zocor, to generic competition next year. Zocor is projected to post sales of $4.4 billion in 2005, according to analysts. And the company stands to lose a $2 billion performer, Fosamax, in 2008.
COPYRIGHT 2005 Reproduced with permission of the copyright holder. Further reproduction or distribution is prohibited without permission.
COPYRIGHT 2005 Gale Group