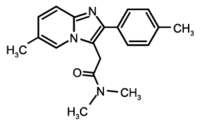
Zolpidem
Zolpidem is a prescription drug used for the short-term treatment of insomnia (sleeping pill). It works quickly (usually within 15 minutes) and has a short half-life (2-3 hours), but will last longer in patients with hepatic failure. Some trade names of zolpidem are Ambien®, Stilnox®, Stilnoct®, or Myslee®. Its sedative effects are similar to those of the benzodiazepines, but it is actually classified as an imidazopyridine, and the anticonvulsant and muscle relaxant effects only appear at 10 and 20 times the dose required for sedation, respectively. more...
For that reason, it has never been approved for either muscle relaxation or seizure prevention. Such drastically increased doses are likely to induce one or more negative side effects, including hallucinations and/or amnesia.
The patent on zolpidem is held by the French pharmacutical corporation Sanofi-Aventis.
Uses
Zolpidem is approved for the short-term treatment of insomnia, but it has been studied for nightly use up to six months in a single-blind, open-label trial published in 1991, an open-label study lasting 180 days published in 1992 (with continued efficacy in patients who had kept taking it as of 180 days after the end of the trial), and in an open-label trial lasting 179 days published in 1993.
The United States Air Force uses zolpidem under trade name Ambien® as "no-go pills" to help the pilots sleep after the mission; another drug used for the same purpose is temazepam (Restoril®). (Cf. the "go-pills", amphetamine served under the name Dexedrine® as a stimulant for the pilots, or its recent modafinil (Provigil®) replacement).
It is also used off-label to treat restless leg syndrome.
As is the case with many prescription sedative/hypnotic drugs, zolpidem is sometimes used by stimulant users to "come down" after the use of stimulants such as methamphetamine, cocaine, methylenedioxymethamphetamine (MDMA), or pharmaceutical amphetamines.
Mechanism of action
In 1990, Pritchett and Seeburg noted that zolpidem binds with high affinity to the α1-, and with medium affinity to the α2- and α3-GABAA receptor subunits, and found that it had no affinity for the α5 subunit. Two years later, zolpidem was noted to have a high affinity for ω1-benzodiazepine receptors, a low affinity for ω2 and a very low affintity for ω3, respectively by Ruano et al in 1992. In other words, it has the highest affinity for ω1 binding sites on α-1GABAA receptor subunits, and it is this that mediates its sedative and weak anticonvulsant properties.
Read more at Wikipedia.org