Zyrtec
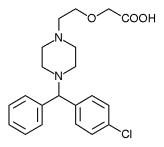
Cetirizine hydrochloride is a medication used for the treatment of allergies, hay fever, angioedema, and hives. It is a second-generation H1-receptor antagonist antihistamine and works by blocking H1 histamine receptors. It is a major metabolite of hydroxyzine, and has the same basic side effects, including dry mouth. more...
- It has long duration of action
- No reported cardiac toxicity associated with the use of this drug
- Minimal penetration of the blood-brain barrier
- Only mild sedating effects, although more than some other non-sedating antihistamines
The medication is produced by UCB, a Belgian pharmaceutical company. The drug is marketed under the following brand names: Zyrtec® in the USA, Zirtek® in the United Kingdom, Zyrlex® in many other European countries, Reactine® in Canada (all by Pfizer ), and as Virlix® in Mexico and parts of Europe (by GlaxoSmithKline ). It can be found under a variety of other brand names in other countries .
Like many other antihistamine medications, cetirizine is commonly prescribed in combination with pseudoephedrine hydrochloride, a decongestant. These combinations are marketed using the same brand name as the cetirizine with a "-D" suffix (Zyrtec-D®, Virlix-D®, etc.)
Read more at Wikipedia.org