ABSTRACT
It was recently postulated that the effects of general anesthetics on protein global dynamics might underlie a unitary molecular mechanism of general anesthesia. To verify that the specific dynamics effects caused by general anesthetics were not shared by nonanesthetic molecules, two parallel 8-ns all-atom molecular dynamics simulations were performed on a gramicidin A (gA) channel in a fully hydrated dimyristoylphosphatidylcholine membrane in the presence and absence of hexafluoroethane (HFE), which structurally resembles the potent anesthetic molecule halothane but produces no anesthesia. Similar to halothane, HFE had no measurable effects on the gA channel structure. In contrast to halothane, HFE produced no significant changes in the gA channel dynamics. The difference between halothane and HFE on channel dynamics can be attributed to their distinctly different distributions within the lipid bilayer and consequently to the different interactions of the anesthetic and the nonanesthetic molecules with the channel-anchoring tryptophan residues. The study further supports the notion that anesthetic-induced changes in protein global dynamics may play an important role in mediating anesthetic actions on proteins.
INTRODUCTION
One of the greatest challenges to the scientific community in the past 150 years is to understand the action of a class of seemingly nonspecific drugs on the central nervous system to produce the physiological state of mind referred to as "general anesthesia". The conceptual difficulties have been the lack of a functional definition of general anesthesia at the molecular level. A significant amount of effort has been devoted in recent years to the identification of anesthetic targets in the central nervous system. Departing from the classical view of lipid perturbation theory, contemporary experiments involving mutagenesis, photoaffinity labeling, and electrophysiology have focused on several anesthetic-sensitive neuronal ion channels (Campagna et al., 2003; Franks and Lieb, 1994) or even anesthetic-sensitive mutation sites in these channels (Forman et al., 1995; Jenkins et al., 2001; Mascia et al., 2000; Mihic et al., 1997; Pratt et al., 2000). The lack of high-resolution structures of these ion channels, however, has impeded the progress in finding out where the anesthetic interaction sites are and, more importantly, how the interactions alter the biological functions of these channels.
High-resolution structures of these ion channel proteins, once resolved, will definitely provide an important structural basis for the interpretation of their responses to general anesthetics. Given the low affinity (K^sub d^ in the sub-mM to mM range) of most general anesthetics at their putative binding sites, however, it has been questioned whether the structure-function paradigm alone is sufficient to explain the molecular mechanisms of general anesthesia (Tang and Xu, 2002). There is a growing realization that protein dynamics plays vital roles in protein functions. Because protein molecules are dynamic in nature, the conventional static view of protein structures can only provide a limited, and often incomplete, understanding of protein functions. For example, the function of myoglobin was viewed for a long time as storage of dioxygen at the heme iron based on the static myoglobin structure (Kendrew et al., 1958; Perutz, 1979). Not until recently, after dynamical aspects of myoglobin were well characterized, did its role other than O2 storage start to emerge. Without integrating the dynamic properties of myoglobin, one could not even clearly pinpoint the pathway for dioxygen to enter the protein because the pathway is not apparent in the static structure (Case and Karplus, 1979; Perutz and Mathews, 1966). Systematic measurements on myoglobin dynamics have confirmed the existence of its conformational substates and identified the importance of conformational substates to the protein functions (Bourgeois et al., 2003; Frauenfelder et al., 2003; Srajer et al., 2001). If dynamics plays such an important role in a monomeric protein like myoglobin, dynamical motion must be essential to the functions of the multisubunit neuronal ion channels. In fact, different functional states of neuronal ion channels, including the open, closed, and slow and fast desensitized states, have already been identified experimentally (Auerbach and Akk, 1998; Karlin, 2002; Neubig et al., 1982). These functionally distinct states likely resulted from equilibrium shift among different conformational substates.
Can general anesthetics modulate different conformational substates or shift the dynamic population of the substates to exert their actions on neuronal ion channels? An unambiguous experimental proof with neuronal ion channels remains a challenge, but the results from our previous molecular dynamics (MD) simulations of the effects of halothane, a potent volatile anesthetic, on gramicidin A (gA) channels have encouraged thinking along this line (Tang and Xu, 2002). Although the anesthetic effect on the structure of the gA channel is minimal, which is consistent with our earlier experimental findings (Tang et al., 1999a, 2000a, 2002), the presence of halothane profoundly affects the channel dynamics, as evidenced by the changes in the root mean-square fluctuation (RMSF) and the autocorrelation function of the gA backbone in the lipid core in the presence of halothane, even though halothane preferentially targets the anchoring residues at the channel-lipid-water interface. Our earlier simulation results discounted the viewpoint that overrates the importance of structural fitting between anesthetic molecules and yet-unidentified hydrophobic protein pockets. Instead, the results suggest at least two important possibilities: 1), direct anesthetic interactions with some of the key residues of ion channel proteins, such as tryptophans in gA, can modulate the dynamics of residues that are remote from anesthetic interaction sites; and 2), protein global dynamics might be crucial for anesthetic action. We hypothesize that drugs such as general anesthetics and alcohols with low affinity binding to proteins can still change protein function specifically by modulating protein global dynamics on various timescales. Whereas multiple conformational substates coexist dynamically for all proteins, the presence of general anesthetics at certain crucial locations within or around the protein can shift the equilibrium among different substates. When anesthetic modulations of the global dynamics of a given protein create "conformation resonance" where one of the equilibrating conformers becomes the dominating conformation, then the function earned out by the protein can potentially be changed. The enhancement of the protein dynamical motion having the characteristic time matching the timescale for the protein function will lead to anesthetic-induced potentiation, whereas matching other motion time constants will lead to anesthetic-induced inhibition or desensitization.
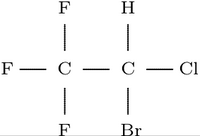
In this study, we test the aforementioned hypothesis using a negative control by replacing anesthetic halothane (CF^sub 3^CHClBr) with nonanesthetic hexafluoroethane (HFE; C^sub 2^F^sub 6^) in the previously studied simulation system (Tang and Xu, 2002). Two parallel ~8-ns MD simulations were performed to investigate if this nonanesthetic molecule can produce the same effects as halothane on the gA channel. The comparison of the effects of structurally similar anestheticnonanesthetic pairs on the same ion channel will elucidate the critical properties that are relevant to the underlying mechanisms of the action of general anesthetics. This study confirmed that the profound changes in gramicidin backbone dynamics occurred only with anesthetic halothane and not with nonanesthetic HFE.
METHODS
Simulation systems
Detailed procedures for the preparation of simulation systems have been reported previously (Tang and Xu, 2002). Two parallel systems, in the absence and presence of HFE, were prepared using the NAMD2 (Kale et al., 1999) and X-PLOR (Brünger, 1992) programs. Each system had a gA channel (1MAG; Ketchem et al., 1997) in the preequilibrated dimyristoyl-phosphatidylcholine (DMPC) membrane, consisting of 182 DMPC lipid molecules fully hydrated with 5538 water molecules (Zubrzycki et al., 2000). The CHARMM22 force field (Brooks et al., 1983; MacKerell et al., 1998) and TIP3P water model (Jorgensen et al., 1983) were used in the study. The geometry of HFE molecules were optimized at the B3LYP/6-311+G(2d,p) level, and the nonbonded interaction parameters of HFE were optimized to be compatible with the CHARMM force field (Liu et al., 2004). For the system with HFE, 10 HFE molecules were placed at the same initial locations as for halothane in the previous study (Tang and Xu, 2002). Additional energy minimization was performed on the system after introducing HFE. The heavy atoms of the gA channel backbone were restrained with a harmonic force during the initial steps of energy minimization to ensure a stable channel structure. The force constant of the restraint was initially set at 999 kcal/mol/[Angstrom]^sup 2^ and was reduced in a stepwise fashion to 0 kcal/mol/[Angstrom]^sup 2^. Both energy-minimized systems, in the absence and presence of HFE, went through equilibration under NVT (constant number of atoms, volume, and temperature) and NPT (constant number of atoms, pressure, and temperature) conditions for 40 ps and 250 ps, respectively, with a harmonic constraint of 0.5 kcal/mol/[Angstrom]^sup 2^ on gA backbone heavy atoms.
MD simulations
After system preparations, two NPT simulations were carried out for 8 ns each in parallel in the presence and absence of 10 HFE molecules on the T3E parallel supercomputer at the Pittsburgh Supercomputing Center using the NAMD2 program. The Nosé-Hoover method with Langevin dynamics and Langevin piston pressure were applied to control the temperature at 305 K (Hoover, 1985; Nose, 1984) and pressure at 1 bar (Feller et al., 1995; Martyna et al., 1994), respectively. The periodic boundary condition was imposed on a flexible cell of an initial dimension of 80 × 80 × 60 [Angstrom]^sup 3^ with water wrapping. The time step was 1 fs for the first 3-ns simulations and was extended to 2 fs for the rest of the simulations. The energies and trajectories were stored every 0.5 and 1 ps, respectively. The cutoff distance for the van der Waals interaction was 12 [Angstrom] with the pair list distance extended to 13 [Angstrom]. The pair list for nonbonded interactions were updated every 20 time steps. The long-range full electrostatic interactions were evaluated every four time steps using the particle-mesh Ewald (PME) method with a PME tolerance of 10^sup -6^ and PME interpolation order of 4 (Darden et al., 1993). The SHAKE routine was used to restrain all bonds between hydrogen and its parent atom to a tolerance of 10^sup -5^ [Angstrom] (Van Gunsteren and Berendsen, 1977) in all simulations.
RESULTS AND DISCUSSIONS
HFE distribution in the gA-membrane system
To compare directly with the halothane results, HFE molecules were purposely placed at the same initial locations as the halothane molecules in our previous simulation system (Tang and Xu, 2002). This initial placement is consistent with our prior experimental knowledge (Tang et al., 1999a) of halothane distribution in the membrane but somewhat artificial for HFE, thus allowing for the specificity of anesthetic and nonanesthetic interaction with the channel protein to be examined. Fig. 1 shows the movement trajectories of HFE molecules in the 8-ns NPT simulation. The trajectories were generated by connecting positions of the center of mass of HFE molecules at each saved time point (every picosecond). The density of the trajectory lines reflected how frequently a HFE molecule sampled a particular region. As shown in Fig. 1 A, all 10 HFE molecules had considerable movement over the 8-ns simulation, as reflected by the large regions encompassed by each motion trajectory. There is a clear tendency for HFE molecules to finally locate in the lipid alkyl-tail region regardless of their initial positions, which were highlighted in Fig. 1 A using HFE molecules in Corey-Pauling-Koltun presentations. A closer examination of the trajectories showed that only some of the HFE molecules (No. 4, No. 5, No. 7, No. 8, and No. 10) had large displacement along the membrane normal direction. Most of these molecules were initially placed near the lipidwatcr interface. Instead of moving around at the interface or moving into the bulk water as their halothane counterparts did in the previous simulations (Tang and Xu, 2002), these HFE molecules moved toward the inner lipid bilayer and showed their preference for the hydrophobic environment. The difference in water solubility between halothane and HFE is also evident: HFE No. 9 and No. 10 in Fig. 1 A moved into and remained within the lipid interior during the entire 8-ns simulations, whereas previously (Tang and Xu, 2002) halothane molecules placed at the corresponding initial positions moved into the lipid-water interface and the bulk water in the 2.2-ns simulations. Thus, unlike anesthetic halothane, none of the nonanesthetic HFE showed an appreciable amount of time in water during the 8-ns simulation. The preference of HFE molecules for the lipid tail region was revealed more clearly in Fig. 1 B, where the displacement of HFE molecules along the z axis (parallel to the membrane normal) was determined. At least 4 out of 10 HFE molecules (No. 4, No. 5, No. 8, and No. 10) were either close to or at the lipid-water interface at the beginning of the simulation, but all of them moved away from the interface and into the lipid interior at the end of the simulation. The maximum z coordinate difference of the 10 HFE molecules was reduced from ~30 [Angstrom] to
The differences between halothane and HFE molecules observed in our simulation systems are consistent with the finding from previous experimental and simulation studies. Both NMR measurements (North and Cafiso, 1997; Tang et al., 1997) and MD simulations (Koubi et al., 2001, 2002; Tu et al., 1998) indicated that anesthetic and nonanesthetic molecules had different preferential localizations and properties in pure lipid membrane. The former preferred amphiphilic lipid-water interfacial regions, whereas the latter resided primarily within the membrane hydrocarbon core. Their difference in effective concentrations at different submolecular sites might account for their ability to target functionally crucial domains in channel proteins and ultimately for their ability to produce general anesthesia. By its Hydrophobic nature, HFE has much less probability than halothane to interact with the anchoring residues of the gA channel near the lipid-water interface. Such interactions in the presence of halothane, observed by NMR (Tang et al., 2000a) and MD simulations (Tang and Xu, 2002), have shown strong effects on the hydrogen bonding between tryptophan side chains and lipids, and consequently on the dynamics behavior of the gA channel.
HFE effects on gA channel structure
The structural stability of gA channel in the presence and absence of HFE was evaluated by root mean-square deviations (RMSDs) and presented in Fig. 2. The presence of nonanesthetic HFE had little impact on the secondary and tertiary structures of the channel. The same conclusion was drawn in the previous study of a gA channel in DMPC in the presence of anesthetic halothane (Tang and Xu, 2002). These simulation results are consistent with our earlier finding from NMR experiments (Tang et al., 1999b, 2002) that anesthetics and their nonanesthetic analogs, in general, have no significant effects on gA channel structures.
HFE effects on the dynamics behavior of gA channel
It was found previously that halothanc caused profound changes in gA channel dynamics (Tang and Xu, 2002). Moreover, anesthetic effects on dynamics varied with the characteristic times of the dynamical motion. In contrast, the presence of HFE had virtually no influence on the dynamics behavior of the gA channel. Fig. 3 compares the RMSF of the gA backbone C^sub α^ in the presence and absence of HFE. To correct the baseline of RMSF by removing the effects of any possible accumulative translational movement of the system, the center of mass of the entire system at all saved points was fitted to a common reference point. As expected, the residues near the entrance of the channel have greater fluctuation than the residues near the center of the channel, forming a U-shaped RMSF profile along the channel. Unlike the system with halothane in which halothane seems to equalize the fluctuation along the channel (Tang and Xu, 2002), this U-shaped profile is well preserved after the addition of HFE to the system, suggesting that HFE does not impose any sizeable effects on the gA channel dynamics.
The autocorrelation function, C^sub i^(t), of the channel backbone N-H vectors gives a more detailed description of the time dependence of the channel dynamics. For display clarity, autocorrelation functions for residues 1-8 and 9-15 were pooled and averaged separately and shown in Fig. 4. Grouping the inner and outer residues allows for possible difference of HFE effects on the anchoring and central part of the channel to be analyzed separately. In both the control and HFE simulations, C^sub i^(t) drops to ~0.9 within picoseconds for all residues, due to the ultrafast subpicosecond libration motion of the N-H vectors. Inner channel residues (1-8) remain at relatively high asymptotic values of ~0.88, whereas overall C^sub i^(t) values for outer residues (9-15) are just slightly lower (~0.82), indicating that the N-H vectors of both inner and outer residues are well ordered on a picosecond time-scale. The variations of C^sub i^(t) between the control and HFE simulations are so trivial that they must be regarded as random fluctuations. Overall, the autocorrelation function data suggested again that HFE exert no significant effects on the dynamics of the channel.
Significance of these results is at least twofold. First, it confirms that the previously observed changes in the gA channel dynamics in the presence of halothane (Tang and Xu, 2002) are indeed caused by the anesthetic interactions with the channel and not fortuitous events. Artificially replacing the anesthetics with nonanesthetics at the same initial positions cannot reproduce the anesthetic effects on protein global dynamics, suggesting that the interactions are anesthetic specific. second, the ability of halothane and possibly other anesthetics to affect the channel dynamics and the inability of nonanesthetic HFE to do the same may represent the most fundamental difference between anesthetics and nonanesthetics in exerting their distinct actions on ion channels.
An important remaining question is why anesthetic can affect gA channel dynamics but structurally similar nonanesthetic cannot. The tryptophan indole amide hydrogen atoms of gA have been found to form stable hydrogen bonds with the phosphate oxygen in the lipid head region or the fatty acid oxygen near the glycerol bridge, depending on the depths of the indoles in the membrane (Tang and Xu, 2002). These hydrogen bonds could be disrupted frequently in the presence of nearby halothane molecules due to the replacement of the channel-membrane hydrogen bonding with hydrogen bonding between the indole amide hydrogen and the fluorine in halothane. Considering there are four tryptophans (W9, W11, W13, and W15) at each end of a gA channel to anchor the channel dimer, it is conceivable that this type of disruption between the anchoring residues and membrane headgroups could affect the entire channel motion (Tang and Xu, 2002) and consequently the channel stability and conductance (Hu et al., 1993; Ketchem et al., 1997) in the membranes. In contrast, nonanesthetic HFE has little effect on disrupting the association of anchoring tryptophan residues with the lipid-water interface. The lack of HFE interaction with membrane interfacial tryptophan residues is clearly a direct consequence of the preferred HFE partitioning in the hydrophobic lipid core of the membrane.
HFE effects on lipids
Lipids play important roles in the stability and function of ion channels. Modification of lipid properties may potentially affect channel behavior. Previous MD simulations (Koubi et al., 2002) on the pure lipid systems showed that the HFE molecules were almost evenly distributed along the lipid hydrocarbon chains with only a slight preference for the bilayer center, which is consistent with the observations in this study (see Fig. 1). The presence of HFE in pure lipid imposed little change on the electrostatic potential across the membrane interface and on the structural and dynamical properties of the lipid core (Koubi et al., 2001), whereas the presence of halothane could cause profound changes to these properties. Contrary to a decrease of the membrane thickness and an increase of the average area per lipid induced by the presence of anesthetic halothane (Koubi et al., 2000; Tu et al., 1998), opposite changes were observed previously in pure lipid systems in the presence of HFE. Comparing to the control system in this study, the system involved with HFE has greater values on membrane thickness and smaller values on the averaged area per lipid (data not shown). The observation is consistent with the findings in the pure lipid system (Koubi et al., 2001), but the extent of the changes is smaller in this study, presumably because of a lower HFE concentration in our system.
The dynamical properties of the lipids in the vicinity of the gA channel are crucial to the channel functions and are evaluated using the order parameters of the lipid alkyl chain. As depicted in Fig. 5, the order parameters are gradually reduced from the headgroup to the alkyl tail in both sn-1 and sn-2 chains. The magnitudes of order parameters for the lipids in the immediate vicinity of the gA channel (i.e., the interfacial boundary lipids) are higher in the headgroup and lower in the tail region than the corresponding values of bulk lipids from earlier experimental and simulation studies (Boden et al., 1991; Douliez et al, 1995; Moore et al., 2001). The increased order near the headgroup can be attributed to the four tryptophan residues at each side of membrane-water interface. The bulky tryptophan side chains served as anchors to stabilize the channel in the membrane, and in doing so, they might at same time produce similar effects to what cholesterols do to rigidify the headgroup of the lipid. Previous NMR experiments (Rice and Oldfield, 1979) indicated the same possibilities. Because the effect of HFE on the lipid dynamics is essentially nonexistent, the variations in the boundary lipid order parameters by HFE were smaller than error bars, suggesting that the presence of ~5% mole fraction of HFE in lipid bilayer has essentially no impact on the lipid acyl chain conformations. A similar result was also obtained from MD simulations (Koubi et al., 2002) of DMPC membrane with mole fraction of HFE up to 25%, indicating the extreme insensitivity of the lipid acyl chain conformations to HFE.
Using a well-defined thermodynamic argument on the basis of membrane lateral pressure, Cantor investigated the lipid-mediated anesthetic effects on ion channels (Cantor, 1997) and predicted that incorporation of amphiphilic and other interfacially active solutes into the bilayer would selectively increase the lateral pressure near the aqueous interfaces, thereby shift the conformational equilibrium of a multidomain channel protein to favor a closed state. He also predicted that perfluorocarbons having low accessibility to the interface would not be able to increase the membrane lateral pressure at the interface, but rather cause a gradual change in lateral pressure profile. Our simulation results are consistent with this prediction and show that no HFE effects can be found on the ordering (or stiffness) of the lipid head-and tail groups.
Finally, it should be noted that the total simulation time in this study is almost four times longer than our previous simulation with halothane. For the properties that can be assessed on the basis of a few nanosecond simulations, 3-ns and 8-ns seem to make no significant difference. Indeed, the same conclusions can be drawn based only on the first 3 ns of simulation in this study. Availability of more computing power than merely 2 years ago allowed us to confirm this assertion by extending the simulation to 8 ns. Neither 2.2-ns simulation nor 8-ns simulation is long enough to study some other interesting properties (e.g., anesthetic effects on ion permeation) that are not attempted in this study.
CONCLUSIONS
Similar to anesthetic halothane (Tang et al., 2000b), nonanesthetic HFE molecules disturbed neither secondary nor tertiary structures of gA channel in the duration of 8-ns simulations. Combining the information obtained in this study with the knowledge acquired before (Bhattacharya et al., 2000; Tang et al., 1999b, 2000a; Tang and Xu, 2002), it can be concluded that low affinity agents, such as volatile anesthetics, are likely to exert their action on proteins without strong perturbation to the protein structures.
The distribution of HFE along membrane normal at the end of an 8-ns simulation clearly shows higher occurrence of HFE toward the tail region of lipids. In contrast, most of halothane molecules moved to the lipid-water interface after 2-ns simulations (Tang and Xu, 2002). Experimentally, we have compared the distributions of anesthetic-nonanesthetic pairs within the membrane (Tang et al., 1997), their interactions with gA channels (Tang et al., 1999a, 2000a), and their effects on the channel structure (Tang et al., 1999b, 2002) and on channel function as measured by the unidirectional Na+ permeation rates (Tang et al., 1999a). The same tendency in membrane distribution and in different interactions with the anchoring residues found in this study as in the previous experiments suggests the high likelihood that halothane and HFE will affect the channel function differently. The lack of HFE at the lipid-water interface precludes HFE from interacting with the channel-anchoring tryptophans or disrupting the hydrogen bonds between tryptophans and the surrounding lipids. Consequently, the channel dynamics was not affected by the presence of HFE. The immediate implication of the finding is that the dynamics changes of gA channel in our previous study in the presence of halothane (Tang and Xu, 2002) are due specifically to the anesthetic interaction with the anchoring residues at the membrane-water interface. Although both halothane and HFE have significant distribution in the lipid tail region near the channel segments deeply embedded in the lipid core, the local nonspecific perturbation is not sufficient to account for the dynamics changes in the middle segments of the gA channel seen in the presence of halothane but not seen in the presence of HFE. Thus, the discriminating property that differentiates the anesthetic effects from the nonanesthetic effects in our previous and these simulations is the ability to modulate the global, as oppose to local, dynamics of the channel proteins. This realization may prove to be crucial for a better understanding of the action of a wide variety of low-affinity drugs on proteins.
This research was facilitated through an allocation of advanced computing resources at the Pittsburgh Supercompuling Center through the support of the National Science Foundation and the Commonwealth of Pennsylvania. This research was supported in part by grants from the National Institutes of Health (R01GM66358, R01GM56257, and R01GM49202).
REFERENCES
Aucrbach, A., and G. Akk. 1998. Desensitization of mouse nicotinic acetylcholine receptor channels. A two-gale mechanism. J. Gen. Physiol. 112:181-197.
Bhatlacharya, A. A., S. Curry, and N. P. Franks. 2000. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J. Biol. Chem. 275:38731-38738.
Boden, N., S. A. Jones, and F. Sixl. 1991. On the use of deuterium nuclear magnetic resonance as a probe of chain packing in lipicl bilayers. Biochemistry. 30:2146-2155.
Bourgeois, D., B. Vallone, F. Schotte, A. Arcovito, A. E. Miele, G. Sciara, M. Wulff, P. Anfinrud, and M. Brunori. 2003. Complex landscape of protein structural dynamics unveiled by nanosecond Laue crystallography. Proc. Natl. Acad. Sci. USA. 100:8704-8709.
Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, and M. Karplus. 1983. CHARMm: a program for macromolecular energy, minimization and dynamics calculations. J. Comput. Chem. 4:187-217.
Brünger, A. T. 1992. X-PLOR, Version 3.1: a system for x-ray crystallography and NMR. Yale University Press, New Haven.
Campagna, J. A., K. W. Miller, and S. A. Forman. 2003. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348:2110-2124.
Cantor, R. S. 1997. The lateral pressure profile in membranes: a physical mechanism of general anesthesia. Biochemistry. 36:2339-2344.
Case, D. A., and M. Karplus. 1979. Dynamics of ligand binding to heme proteins. J. Mol. Biol. 132:343-368.
Dardcn, T., D. York, and L. Pedersen. 1993. Particle Mesh Ewald-an N. Log(N) method for Ewald sums in large systems. J. Client. Phys. 98:10089-10092.
Douliez, J. P., A. Leonard, and E. J. Dufourc. 1995. Restatement of order parameters in biomembranes: calculation of C-C bond order parameters from C-D quadrupoiar splittings. Biophys. J. 68:1727-1739.
Feller, S. E., Y. H. Zhang, R. W. Pastor, and B. R. Brooks. 1995. Constant-pressure molecular-dynamics simulation-the Langevin piston method. J. Chew. Phys. 103:4613-4621.
Forman, S. A., K. W. Miller, and G. Yellen. 1995. A discrete site for general anesthetics on a postsynaplic receptor. Mol. Pharmacol. 48:574-581.
Franks, N. P., and W. R. Lieb. 1994. Molecular and cellular mechanisms of general-anesthesia. Nature. 367:607-614.
Frauenfelder, H., B. H. McMahon, and P. W. Fenimore. 2003. Myoglobin: the hydrogen atom of biology and a paradigm of complexity. Prof. Natl. Acad. Sci. USA. 100:8615-8617.
Hoover, W. G. 1985. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. 31:1695-1697.
Hu, W., K. C. Lee, and T. A. Cross. 1993. Tryplophans in membrane proteins: indole ring orientations and functional implications in the gramicidin channel. Biochemistry. 32:7035-7047.
Huang, P., J. J. Perez, and G. H. Loew. 1994. Molecular dynamics simulations of phospholipid bilayers. J. Biomol. Struct. Dyn. 11:927-956.
Humphrey, W., A. Dalke, and K. Schulten. 1996. VMD: visual molecular dynamics. J. Mol. Graph. 14:33-38.
Jenkins, A., E. P. Greenblatt, H. J. Faulkner, E. Bertaccini, A. Light, A. Lin, A. Andreasen, A. Viner, J. R. Trudell, and N. L. Harrison. 2001. Evidence for a common binding cavity for three general anesthetics within the GABAA receptor. J. Neurosci. 21:RC136:1-4.
Jorgensen, W. L., J. Chandrasekhar, J. D. Madura, R. W. Impey, and M. L. Klein. 1983. Comparison of simple potential functions for simulating liquid water. J. Client. Phys. 79:926-935.
Kale, L., R. Skeel, M. Bhandarkar, R. Brunner, A. Gursoy, N. Krawetz, J. Phillips, A. Shinozaki, K. Varadarajan, and K. Schulten. 1999. NAMD2: greater scalability for parallel molecular dynamics. J. Comput. Phys. 151:283-312.
Karlin, A. 2002. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 3:102-114.
Kendrew, J. C., G. Bodo, H. M. Dintzis, R. G. Parrish, H. Wyckoff, and D. C. Phillips. 1958. A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. Nature. 181:662-666.
Ketchem, R., B. Roux, and T. Cross. 1997. High-resolution polypeptide structure in a lamellar phase lipid environment from solid state NMR derived orientational constraints. Structure. 5:1655-1669.
Koubi, L., M. Tarek, S. Bandyopadhyay, M. L. Klein, and D. Scharf. 2001. Membrane structural perturbations caused by anesthetics and nonimmobilizers: a molecular dynamics investigation. Biophys. J. 81:3339-3345.
Koubi, L., M. Tarek, S. Bandyopadhyay, M. L. Klein, and D. Scharf. 2002. Effects of the nonimmobilizer hexafluroethane on the model membrane dimyristoylphosphatidylcholine. Anesthesiology. 97:848-855.
Koubi, L., M. Tarek, M. L. Klein, and D. Scharf. 2000. Distribution of halothanc in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys. J. 78:800-811.
Liu, Z. W., Y. Xu, A. C. Saladino, T. Wymore, and P. Tang. 2004. Parametrization of 2-bromo-2-chloro-1,1,1-trifluoroethane (halothane) and hexafluoroethane for nonbonded interactions. J. Phvs. Chem. A. 108:781-786.
MacKerell, A. D., D. Bashford, M. Bellott, R. L. Dunbrack, J. D. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo, S. Ha, D. Joseph-McCarthy, L. Kuchnir, and others. 1998. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 102:3586-3616.
Martyna, G. J., D. J. Tobias, and M. L. Klein. 1994. Constant-pressure molecular-dynamics algorithms. J. Chem. Phys. 101:4177-4189.
Mascia, M. P., J. R. Trudell, and R. A. Harris. 2000. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc. Natl. Acad. Sci. USA. 97:9305-9310.
Mihic, S. J., Q. Ye, M. J. Wick, V. V. Koltchine, M. D. Krasowski, S. E. Finn, M. P. Mascia, C. F. Valenzuela, K. K. Hanson, E. P. Greenblatt, R. A. Harris, and N. L. Harrison. 1997. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 389:385-389.
Moore, P. B., C. F. Lopez, and M. L. Klein. 2001. Dynamical properties of a hydrated lipid bilayer from a multinanosecond molecular dynamics simulation. Biophys. J. 81:2484-2494.
Neubig, R. R., N. D. Boyd, and J. B. Cohen. 1982. Conformations of Torpedo acetylcholine receptor associated with ion transport and desensitization. Biochemistry. 21:3460-3467.
North, C., and D. S. Cafiso. 1997. Contrasting membrane localization and behavior of halogenated cyclobutanes that follow or violate the Meyer-Overton hypothesis of general anesthetic potency. Biophys. J. 72:1754-1761.
Nose, S. 1984. A unified formulation of the constant-temperature molecular-dynamics methods. J. Chem. Phys. 81:511-519.
Perutz. M. F. 1979. Regulation of oxygen affinity of hemoglobin: influence of structure of the glohin on the heme iron. Annn. Rev. Biochem. 48:327-386.
Perutz, M. F., and F. S. Mathews. 1966. An x-ray study of azide methaemoglobin. J. Mol. Biol. 21:199-202.
Pratt, M. B., S. S. Husain, K. W. Miller, and J. B. Cohen. 2000. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatiblc general anesthetic. J. Biol. Chem. 275: 29441-29451.
Rice, D., and E. Oldfield. 1979. Deuterium nuclear magnetic resonance studies of the interaction between dimyristoylphosphatidylcholine and gramicidin A'. Biochemistry. 18:3272-3279.
Srajer, V., Z. Ren, T. Y. Teng, M. Schmidt, T. Ursby, D. Bourgeois, C. Pradervand, W. Schildkamp, M. Wulff, and K. Moffat. 2001. Protein conformational relaxation and ligand migration in myoglobin: a nanosecond to millisecond molecular movie from time-resolved Laue x-ray diffraction. Biochemistry. 40:13802-13815.
Tang, P., R. G. Eckenhoff, and Y. Xu. 2000a. General anesthetic binding to gramicidin A: the structural requirements. Biophys. J. 78:1804-1809.
Tang, P., J. Hu, S. Liachenko, and Y. Xu. 1999a. Distinctly different interactions of anesthetic and nonimmobilizer with transmembrane channel peptides. Biophys. J. 77:739-746.
Tang, P., P. K. Mandai, and M. Zegarra. 2002. Effects of volatile anesthetic on channel structure of gramicidin a. Biophys. J. 83:1413-1420.
Tang, P., V. Simplaceanu, and Y. Xu. 1999b. Structural consequences of anesthetic and nonimmobilizer interaction with gramicidin A channels. Biophys. J. 76:2346-2350.
Tang, P., and Y. Xu. 2002. Large-scale molecular dynamics simulations of general anesthetic effects on the ion channel in the fully hydrated membrane: the implication of molecular mechanisms of general anesthesia. Proc. Natl. Acad. Sci. USA. 99:16035-16040.
Tang, P., B. Yan, and Y. Xu. 1997. Different distribution of lluorinated anesthetics and nonanesthetics in model membrane: a 19F NMR study. Biophys. J. 72:1676-1682.
Tang, Y. Z., W. Z. Chen, and C. X. Wang. 2000b. Molecular dynamics simulations of the gramicidin A-dimyristoylphosphatidylcholine system with an ion in the channel pore region. Eur. Biophys. J. 29:523-534.
Tu, K., M. Tarek, M. L. Klein, and D. Scharf. 1998. Effects of anesthetics on the structure of a phospholipid bilayer: molecular dynamics investigation of halothane in the hydrated liquid crystal phase of dipalmitoylphosphatidylcholine. Biophys. J. 75:2123-2134.
Van Gunsteren, W. F., and H. J. C. Berendsen. 1977. Algorithms for macromolecular dynamics and constraint dynamics. Mol. Phys. 34:1311-1327.
Zubrzycki, I. Z., Y. Xu, M. Madrid, and P. Tang. 2000. Molecular dynamics simulations of a fully hydrated dimyristoylphosphatidylcholine membrane in liquid-crystalline phase. J. Chem. Phys. 112:3437-3441.
Zhanwu Liu, Yan Xu, and Pei Tang
Department of Anesthesiology and Department of Pharmacology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania 15261
Submitted November 1, 2004, and accepted for publication March 8, 2005.
Address reprint requests to Prof. Pei Tang, W-1357 Biomedical Science Tower, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261. Tel.: 412-383-9798; Fax: 412-648-9587; E-mail: tangp@anes.upmc.edu.
Copyright Biophysical Society Jun 2005
Provided by ProQuest Information and Learning Company. All rights Reserved