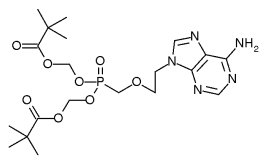
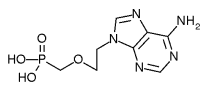
Safety: Adefovir is associated with several toxicities that warrant careful patient monitoring. (1,2) Due to treatment-limiting nephrotoxicity, all patients receiving adefovir need routine monitoring of renal function, especially patients who take other nephrotoxic drugs (e.g., cyclosporine, aminoglycosides) and patients predisposed to renal dysfunction. Lactic acidosis and severe hepatomegaly with steatosis can occur when adefovir is used alone or in combination with other antiretrovirals. Discontinuation of therapy may result in an acute exacerbation of hepatitis. While an exact monitoring interval has not been specified by the manufacturer, patients in clinical trials had monthly monitoring of serum creatinine, phosphorus, and hepatic transaminases. Adefovir is a pregnancy category C. (2)
Tolerability: Side effects are uncommon. The most frequently reported side effects due to adefovir occurred at rates similar to placebo: asthenia (13 percent), headache (9 percent), abdominal pain (9 percent), and nausea (5 percent). (2)
Effectiveness: Adefovir has been studied in several different clinical presentations of hepatitis B. In an unblinded study of 550 patients who were hepatitis B e-antigen-positive, patients were randomized to receive either 10 mg or 30 mg of oral adefovir or placebo. After 48 weeks, both groups receiving adefovir were significantly more likely than the placebo group to have normalization of liver function tests, undetectable HBV DNA levels (21 and 39 percent versus zero percent) and histologic improvement (53 and 59 percent versus 25 percent). The higher dosage was associated with mild renal toxicity. (3) A study of 185 hepatitis B e-antigen-negative patients showed that those receiving adefovir were significantly more likely than the placebo group to have normalization of liver function tests, undetectable HBV DNA levels (51 versus zero percent), and histologic improvement (64 versus 33 percent). (4) Neither study found evidence of adefovir resistance for HBV DNA polymerase. (3,4) In a small case series, adefovir also has been shown to be effective in lamivudine-resistant patients. (5) Adefovir has not been compared directly with lamivudine or with interferon alfa-2b.
Price: A one-month supply of adefovir 10 mg will cost $528 versus $303 for lamivudine.
Simplicity: The dosage of adefovir is one tablet (10 mg) orally daily. The required duration of treatment is unknown. A dosage adjustment is needed for patients with a creatinine clearance <50 mL per min.2 Regular monitoring of renal and hepatic function (as often as monthly) is required. Also, HIV testing should be offered to patients prior to and during adefovir therapy because resistance may result in patients who have undiagnosed or untreated HIV.
Bottom line: Adefovir offers an effective first-line alternative for the treatment of chronic hepatitis B. It also may prove to be useful in patients who have failed therapy with lamivudine as well as in patients who have difficulty tolerating interferon alfa-2b. How it compares with other agents or with combination therapy is unknown.
REFERENCES
(1.) Karayiannis P. Hepatitis B virus: old, new and future approaches to antiviral treatment. J Antimicrob Chemother 2003;51:761-85.
(2.) Package insert. Hepsera. Gilead Sciences, Inc. Accessed november, 2003, at: http://www.hepsera. com/GIL214-07_Hepsera_PDF.8.pdf.
(3.) Marcillin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med 2003; 348:808-16.
(4.) Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003;348:800-7.
(5.) Perrillo R, Schiff E, Yoshida E, et al. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 2000;32:129-34.
STEPS drug updates cover Safety, Tolerability, Effectiveness, Price, and Simplicity.
The series coordinator of STEPS is Allen F. Shaughnessy, Pharm.D., director of medical education at Pinnacle Health System, Harrisburg, Pa.
Monika N. Daftary, Pharm.D., is assistant professor in the Department of Clinical and Administrative Pharmacy Sciences, Howard University School of Pharmacy, Washington, D.C. She is also ambulatory care pharmacist in the outpatient infectious diseases clinic, Howard University Hospital.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group