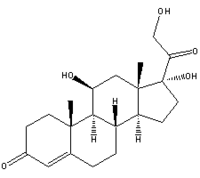
Within the last few years, increasing evidence of relative adrenal insufficiency in septic shock evoked a reassessment of hydrocortisone therapy. To evaluate the effects of hydrocortisone on the balance between proinflammatory and antiinflammation, 40 patients with septic shock were randomized in a double-blind crossover study to receive either the first 100 mg of hydrocortisone as a loading dose and 10 mg per hour until Day 3 (n = 20) or placebo (n = 20), followed by the opposite medication until Day 6. Hydrocortisone infusion induced an increase of mean arterial pressure, systemic vascular resistance, and a decline of heart rate, cardiac index, and norepinephrine requirement. A reduction of plasma nitrite/nitrate indicated inhibition of nitric oxide formation and correlated with a reduction of vasopressor support. The inflammatory response (interleukin-6 and interleukin-8), endothelial (soluble E-selectin) and neutrophil activation (expression of CD1 Ib, CD64), and antiinflammatory response (soluble tumor necrosis factor receptors I and II and interleukin-10) were attenuated. In peripheral blood monocytes, human leukocyte antigen-DR expression was only slightly depressed, whereas in vitro phagocytosis and the monocyteactivating cytokine interleukin-12 increased. Hydrocortisone withdrawal induced hemodynamic and immunologic rebound effects. In conclusion, hydrocortisone therapy restored hemodynamic stability and differentially modulated the immunologic response to stress in a way of antiinflammation rather than immunosuppression.
Keywords: sepsis; glucocorticoids; immune system
During sepsis, the systemic inflammatory response comprises reciprocal communication between the neuroendocrine and the peripheral immune system (1). Proinflammatory mediators recruit the hypothalamic-pituitary-adrenal axis to counter-- regulate inflammation through the synthesis of the stress hormone cortisol. The fundamental roles of glucocorticoids in stress response to infection and increasing knowledge of the antiinflammatory and immunosuppressive pharmacodynamic profile have been the rationale for its use in sepsis trials for decades. Timing, dosage, and duration of glucocorticoid administration were adapted to different disease pathophysiologic models and probably had a major impact on outcome (2). Several randomized controlled trials unequivocally revealed that short-time (1 to 2 days) administration of high doses of glucocorticoids (up to 40 g of hydrocortisone equivalent per day) in early septic shock was without effect on outcome or was even harmful-most probably because of immunosuppression and increased incidence of secondary infections (3, 4). Only one study showed an initial improvement of survival and shock reversal with high-dose methylprednisolone, but with ongoing disease, the differences were no longer significant (5).
In contrast to these former approaches, recent randomized controlled trials indicate that prolonged (5 days or more) administration of "low" doses (compared with the doses above) of hydrocortisone (244-300 mg per day) in early or late septic shock improves shock reversal (6, 7) and outcome (8, 9). These results are in agreement with the concept of impaired adrenocortical reserve in septic shock (2, 10-13). However, the diagnosis and predictive value of relative adrenal insufficiency in septic shock is still a matter of current discussions (14). Although it was reported that shock reversal and outcome were independent from adrenal reserve (8), other reports indicate that the degree of adrenal dysfunction correlates with outcome (15) and that hydrocortisone therapy reduces mortality only in patients with impaired adrenal reserve (9).
Overall, the encouraging results of the recent low-dose hydrocortisone trials evoked a reassessment of the role of glucocorticoids in septic shock. It is well established that glucocorticoids modulate the stress response to sepsis dose dependently through permissive (e.g., enhancing the cardiovascular response to vasopressors) and suppressive (e.g., inhibition of cytokine synthesis) effects (16). However, there is little known about the immunologic effects of continuously infused low doses of hydrocortisone in septic shock. In particular, protection from overshooting inflammatory response has to be weighted against the risk of aggravated immunosuppression, which gained increasing importance in understanding of sepsis pathophysiology and multiple organ failure (17). Because low doses of glucocorticoids are increasingly used as an adjunctive therapy to stabilize blood pressure in septic shock patients, knowledge of immune reactions is of clinical importance to elucidate possible risks accompanied with this therapeutic approach. Therefore, we investigated the effects of a 3-day hydrocortisone treatment on hemodynamic and immunologic parameters, comparing the response with the application and the withdrawal of hydrocortisone in a two-- period crossover design.
METHODS
The study protocol was approved by the institutional ethics committee. Patients who met these criteria were found to be eligible for enrollment: (I) written informed consent from the next of kin; (2 ) the presence of septic shock (18), including (a) proven or strongly suspected infection, (b) three or more of these conditions: mechanical ventilation, heart rate of more than 90 beats per minute, temperature of more than 38 deg C or less than WCC, a white blood cell count of more than 12,000 cells/ (mu)l or less than 4,000 cells/(mu)l, or more than 10% immature cells, and (c) sepsis-induced hypotension (systolic blood pressure of less than 90 mm Hg or a reduction of more than 40 mm Hg from baseline in the absence of other causes of hypotension); and (3) patients requiring norepinephrine to maintain a mean arterial pressure of more than 70 mm Hg despite adequate fluid resuscitation. An age of less than 18 years, glucocorticoid medication within the last 3 months, immunosuppressive therapy, hematologic diseases, pregnancy, and a moribund state were exclusion criteria.
Eligible patients were randomized by the pharmacist into two groups: one group (hydrocortisone group 1 tHC-1]) first received 100 mg of a hydrocortisone loading dose over 30 minutes, followed by 10 mg/ hour until Day 3 and isotonic saline from Days 4 to 6; the other group (HC-2) first received the placebo and then hydrocortisone. Treatment of septic shock patients according to standard regimes of the intensive care unit was not affected by the study protocol (see the online supplement for additional information). Norepinephrine therapy was tapered individually with ongoing hemodynamic stabilization, keeping the mean arterial pressure more than 70 mm Hg. Patients continued to receive standard treatment after Day 6, and in case of ongoing hemodynamic instability, hydrocortisone was administered according to the physician's decision.
Measurements
The average of triple cardiac output measurements (thermodilution technique) was recorded together with norepinephrine requirement at the time of hemodynamic investigation. Mean arterial pressure heart rate, central venous pressure, pulmonary capillary wedge pressure, and mean pulmonary arterial pressure were recorded from online monitoring devices; systemic and pulmonary vascular resistance and cardiac index were calculated with standard formulae.
Cortisol was measured with radioimmunoassay. Interleukin (IL)-4, IL-6, IL-8, IL-10, IL-12 p70, and interferon--y, soluble E-selectin, and soluble tumor necrosis factor receptors I and II were measured with ELISA, and nitrite/nitrate (NOx) was measured with Griess reaction. Leukocyte differentiation, expression of human leukocyte antigen (HLA)-DR on monocytes and of CDllb and CD64 on granulocytes, and in vitro phagocytosis and respiratory burst of monocytes and granulocytes were measured with flow cytometry (see the online supplement for additional information). Blood from 10 healthy individuals was used for control subjects. For assessment of the Simplified Acute Physiology Score 11 (19) and the Sepsis-related Organ Failure Assessment (20), daily recorded data were analyzed.
Statistical Analysis
Statistical procedures are based on recommendations for analysis of crossover trials (21) and included analyses of hydrocortisone-dependent and -independent effects (see Figure 1). Within-group changes of variables for the hydrocortisone early (Days 0-3), hydrocortisone late (Days 3-6), placebo early (Days 0-3), and placebo late (Days 36) periods were analyzed with Friedman repeated-measures analysis of variance on ranks, when indicated. Nominal data proportions were compared with Fisher's exact test. Spearman rank correlation coefficient was calculated to quantify correlations. The false discovery rate procedure (22) revealed a corrected p value of less than 0.02 to be significant for multiple comparisons.
RESULTS
Fifty-nine patients with septic shock were consecutively examined for eligibility between March 1997 and September 2000. Forty patients were enrolled in the study, and 19 patients were not included (13 with steroid history, 1 with hematologic disease, 2 without consent, 1 with extracorporeal membrane oxygenation, and 2 moribund patients). Patients were included within 48 hours after the onset of septic shock or as soon as possible after referral from tertiary hospitals.
Baseline Characteristics
No significant differences between HC-1 and HC-2 were evident at the time of inclusion for age, sex, Simplified Acute Physiology Score II, Sepsis-related Organ Failure Assessment, time between the onset of septic shock and inclusion, the diagnosis of underlying diseases, the source of infection, and bacteriologic results (Table 1). None of the patients died within the observational period of 6 days. Intensive care unit and hospital mortality were 30% in both groups.
HC Infusion Increased Plasma Cortisol Concentration in All Patients
Baseline plasma cortisol was not significantly different between groups, for HC-1 844 (mean) nmol/l (95% confidence interval, 614, 1,074) and for HC-2 822 nmol/l (670, 974). Two patients in the HC-2 group had very low cortisol baseline values; in one of these, cortisol increased spontaneously from 218 to 351 nmol/1 until hydrocortisone treatment; in the other patient, values ranged between 209 and 222 nmoll until hydrocortisone infusion. Both patients finally survived.
Hydrocortisone treatment increased plasma cortisol levels in all patients of both the HC-1 group and the HC-2 group by about factor 5 (1.6- to 15-fold). Peak levels were reached within 1 day after the start of hydrocortisone infusion (means of 3,500 and 3,200 nmol/1 in HC-1 and HC-2, respectively). The individually different increase in plasma cortisol during hydrocortisone treatment was not related to the distinct groups, baseline cortisol, or other patient characteristics. Hydrocortisone withdrawal in HC-I induced a complete reversal of hypercortisolemia to baseline level within 1 day (Figure 1).
Hydrocortisone Improved Hemodynamic Variables and Reduced Plasma NOx The increase in plasma cortisol was accompanied by a significant systemic hemodynamic stabilization, evidenced by an increase of mean arterial pressure and systemic vascular resistance and a concomitant reduction of heart rate and cardiac index. These hemodynamic effects were significantly more pronounced in patients who received hydrocortisone, although norepinephrine requirement in these patients declined. Withdrawal of hydrocortisone on Day 3 had opposite effects on hemodynamic parameters in HC-1. Mean pulmonary arterial pressure, pulmonary vascular resistance, pulmonary capillary wedge pressure, and central venous pressure were not affected by hydrocortisone treatment (Table 2). On Day 3, in HC-1 and HC-2, 14 of 20 and 6 of 20 patients, respectively, were without norepinephrine (p = 0.025); on Day 6, in HC-1 and HC-2, 12 of 20 and 17 of 20 patients, respectively, were weaned from norepinephrine (p = 0.15). In HC-1, 6 of 20 (30%) patients required norepinephrine again after glucocorticoid withdrawal.
Plasma NOx concentrations were reduced during both hydrocortisone treatment periods and remained attenuated after hydrocortisone cessation in HC-1, whereas placebo had no effect on NOx in HC-2. Because NOx remained low in HC-1 after drug withdrawal, a carryover effect was considered, and an additional within-period analysis of variance (Friedman) was performed, yielding significance only during hydrocortisone treatment (Figure 2). At the end of a hydrocortisone-treatment period, patients not requiring norepinephrine had significant lower plasma NOx than patients who required any dose of norepinephrine (25 [27, 42] (mu)mol/l versus 64 [39, 90]; p = 0.015). Plasma NOx concentralions correlated with the norepinephrine requirement (r = 0.34 [0.25, 0.44], p
Hydrocortisone Downregulated Systemic Inflammatory Immune Response but Did Not Inhibit the Th1 -related Response
Hydrocortisone infusion differentially modulated the release of inflammatory mediators and expression of cell adhesion molecules. IL-6 and the chemoattractant cytokine IL-8 were both significantly reduced during hydrocortisone treatment (Figure 3). Almost 24 hours after hydrocortisone initiation, concentrations of both cytokines were significantly lower in HC-1 than in HC-2 (p
Hydrocortisone Inhibited the Antiinflammatory Immune Response
Hydrocortisone infusion modulated release of mediators and expression of receptors, which are associated with an antiinflammatory immune response. IL-10 and soluble tumor necrosis factor receptors I and II were significantly reduced. Again, hydrocortisone withdrawal had opposite effects and induced a significant increase of IL-10 and tumor necrosis factor receptors (p
Hydrocortisone Did Not Induce Severe Monocyte/ Granulocyte Dysfunction
The HLA-DR expression on monocytes is essential for their antigen-presenting activity and is a sensitive marker of monocytic function. Baseline HLA-DR antigen expression, quantified as a percentage of positive monocytes, was significantly depressed compared with values of healthy individuals (more than 85%) but did not reach the level of "immunoparalysis" (less than 30%). Most importantly, the levels were not further reduced within 3 days of hydrocortisone treatment (65 [58, 72] versus 63% [57, 69], 95% CI). However, the more sensitive HLA-DR antigen density analysis expressed as geometric mean fluorescence intensity revealed a marginal loss of receptor density within the first 2 days of treatment. On the other hand, mean fluorescence exceeded control values after HC withdrawal in HC-1 group, thus resulting in an overall significant difference between the HC and placebo phases (Figure 8).
Monocyte phagocytosis ex vivo was enhanced during hydrocortisone treatment, whereas granulocyte phagocytosis was slightly depressed (2%). Granulocyte respiratory burst was not affected by hydrocortisone treatment (see Table El in the online supplement).
DISCUSSION
The purpose of this study was to investigate the effects of a lowdose continuous hydrocortisone infusion on hemodynamic and immunologic variables in patients with septic shock and to determine whether this therapeutic approach bears the risk of severe immunosuppression. Our data demonstrated that low-dose bydrocortisone treatment (1) improved hemodynamic parameters, (2) inhibited systemic inflammation and prevented overwhelming compensatory antiinflammatory response, and (3) maintained Thl-related immune responsiveness.
The presented data are in accordance with other results of hydrocortisone-induced hemodynamic improvement and reduction of vasopressor support in septic shock (6-9, 23-26). Hydrocortisone treatment induced significant changes of the systemic circulation without affecting pulmonary vascular resistance or cardiac filling pressures. The increase of mean arterial pressure was accompanied by a significant reduction of norepinephrine requirement, an increase of systemic vascular resistance, and a reduction of cardiac index and heart rate, implying effects predominantly on vascular tone of resistance vessels. Most patients could be weaned from vasopressor therapy within 2-3 days. However, a significant but less impressive reduction of norepinephrine was also observed with placebo, indicating an improvement of the clinical course independent from hydrocortisone therapy. Overall, shock reversal in hydrocortisone-treated patients was faster than in the placebo group, as reported by others (6, 8). Although HC therapy was shown to reverse septic shock independently from adrenal functional reserve (8), other trials indicate that patients with impaired response to corticotropin are more susceptible to cortisol replacement (26, 27). In this study, abrupt drug withdrawal reinduced norepinephrine dependency in 30% of patients previously weaned from vasopressors. Because we did not perform adrenal function tests, it remains speculative whether shock after hydrocortisone withdrawal correlated with adrenal reserve.
In septic shock, overwhelming production of nitric oxide mediates peripheral vasodilation, catecholamine resistance, tissue hypoxia, and cardiomyopathy (28-30). In our patients, increased plasma NOx formation as the main breakdown product of cytokine-induced nitric oxide metabolism was found to correlate with shock severity, organ failure, and outcome (31-34). However, experimental sepsis models and a recent phase III study with L-arginine analogue N^sup G^-monomethyl-L-arginine strongly indicate that excessive and nonspecific blocking of nitric oxide synthases (NOSs) is fatal, stressing the importance of cytoprotective and regulative properties of maintained basal nitric oxide production (35-37). Therefore, selective inhibition of inducible NOS might be more effective and targets the "pathologic" nitric oxide while leaving the "physiologic" nitric oxide unaffected (35). In contrast to nonspecific competitive inhibitors of nitric oxide synthesis (L-arginine analogues, isothioureas), glucocorticoids inhibit inducible NOS but not the constitutively expressed endothelial NOS (38, 39). Inhibition of nitric oxide formation by glucocorticoids was demonstrated in vitro at different levels: transcription, translation, substrate, or enzyme cofactor availability and calpain-induced inducible NOS degradation (40-43).
The presented data give evidence that inhibition of nitric oxide formation contributed to hemodynamic improvement, although we were unable to find direct correlations between plasma NOx concentrations and hemodynamic variables. This discrepancy might be explained by the study design determining a stable blood pressure by titrating norepinephrine dosage. Indeed, plasma NOx correlated significantly only with norepinephrine requirement, an effect that was even more pronounced during hydrocortisone treatment, indicating that inhibition of nitric oxide formation increased vascular tone. Furthermore, hydrocortisone treatment seemed to maintain basal nitric oxide synthesis, as NOx concentrations were reduced from 70-80 to 30-40 (mu)M, which is in agreement with reported levels in nonseptic control subjects in the order of 30-40 and 60-100 (mu)M in patients with severe septic shock (35). However, the discrepancy between sustained NOx suppression and hemodynamic deterioration in some patients after hydrocortisone cessation emphasizes nitric oxide-independent rebound mechanisms. In fact, the physiologic role of glucocorticoids in regulation of systemic blood pressure includes numerous mechanisms of action: signal transduction, prostaglandin metabolism, sodium and calcium transport, and modulation of adrenoreceptors, angiotensin receptors, endothelin receptors, and mineralocorticoid receptors (44,45). Therefore, the impact of hydrocortisone on vascular tone in septic shock needs further investigation. Taken together, inhibition of inducible NOS by a short-time treatment with hydrocortisone contributed to hemodynamic stabilization, and this approach might be as effective, less expensive, and perhaps safer than the use of competitive NOS inhibitors.
Because glucocorticoids affect the immune system at different levels, possibly influencing the balance between inflammation and antiinflammation, we measured several parameters representing different immune responses.
Overall, the inflammatory response was attenuated by hydrocortisone. The amount of inflammatory markers such as IL-6 and IL-8, which were found to correlate with disease severity and worse outcome (46-50), and soluble E-selectin, reflecting endothelial activation (51-53), was significantly suppressed. Furthermore, circulating neutrophils expressed less markers of activation (complement receptor 3 [CD1 tb/CD18] and high-affinity Fcy-receptor I [CD64]) (54, 55) without a reduction of cell-- bound L-selectin (data not shown), which can be induced by glucocorticoids impairing cell recruitment at the side of inflammation (56-58). Importantly, in vitro granulocyte function (respiratory burst and phagocytosis) remained intact, indicating that low-dose hydrocortisone did not suppress innate defense mechanisms. Our results are in agreement with recent reports of hydrocortisone-induced attenuated systemic inflammatory response, evidenced by reduced phospholipase A^sub 2^, neutrophil elastase, C-reactive protein, and IL-6 plasma concentrations (25, 59).
Overall, hydrocortisone did not induce immunosuppression. Several markers such as IL-10 and soluble tumor necrosis factor receptors decreased during hydrocortisone therapy. Moreover, the inhibition of IL-6 synthesis attenuated the antiinflammatory response, as this cytokine is increasingly recognized as an antiinflammatory mediator (60). A switch from Thl to Th2 cells and overproduction of antiinflammatory cytokines promote the risk of infection and may worsen outcome (61-63). There is some evidence that low-dose hydrocortisone did not promote proliferation of antiinflammatory T cell subsets. The amount of Th2-- derived cytokines did not change (IL-4) or decrease (IL-10), and interferon-y, which is released by Thl and suppressor cells, was not suppressed. Recently, it could be shown that a switch of suppressor cell subsets with increased release of IL-4 and decreased production of IFN-gamma is often found in nonsurvivors after burns (64). Furthermore, in concanavalin A-stimulated whole-blood samples, analyzed for release of IL-4 and IL-10, both antiinflammatory cytokines rather decreased during hydrocortisone infusion. Finally, IL-12, a central regulatory cytokine directing Thl development and monocyte activation, increased during hydrocortisone treatment. This stands in contrast to dosedependent in vitro inhibition of IL-12 synthesis by glucocorticoids in nonseptic conditions (65-67). Dose-dependent promoting properties of glucocorticoids in regulation of host resistance, receptor regulation, and cytokine synthesis are currently debated (68). Therefore, further studies are needed to define the impact of hydrocortisone on proliferation of T cell subsets and cytokine release in septic shock.
Monocytes are crucially involved in adaptive immune responses. The reduced capability of monocytes to present antigens to T cells due to reduction of surface HLA-DR promotes risk of infection and may worsen outcome (69-72). Recent observational studies indicate that interferon-gamma treatment may be advantageous in immunoparalyzed septic shock patients (73). In our study, in vitro monocyte phagocytosis was not impaired during hydrocortisone treatment. However, hydrocortisone further depressed HLA-DR expression, which was already low in patients compared with healthy control subjects. The hydrocortisone-- induced receptor downregulation was transient and limited, followed by a rebound increase of receptor expression immediately after drug withdrawal. Hence, adverse effects have to be considered in severely immunodepressed patients. In early septic shock when inflammation often predominates, hydrocortisone might be safer than in prolonged septic shock with increasing incidence of immunosuppression.
Most of the observed hemodynamic and immune effects were followed by a rebound effect when hydrocortisone was withdrawn. It is therefore recommended that patients be weaned from hydrocortisone over several days to avoid adverse effects.
It may be considered that constant hydrocortisone doses administered in this study had probably not met individual needs and that it could be advantageous to titrate hydrocortisone therapy more individually. Furthermore, our data are only representative for a 3-day observational period of hydrocortisone infusion, and prolonged treatment might have different effects on the immune system. Nevertheless, most patients could be weaned from vasopressors in early septic shock within a few days, which might allow a reduction of the dose, thus minimizing the risk of possible adverse effects with prolonged treatment.
Subgroup analyses revealed no significant difference of hemodynamic or immunologic parameters after 3 days of hydrocortisone therapy between 11 patients who received hydrocortisone in early septic shock (less than 48 hours) compared with 29 patients who were treated in late septic shock (more than 48 hours).
Whether the distinct effects of hydrocortisone on the immunologic profile, demonstrated in this study, finally result in a better outcome could not be answered because measurements were performed within a 6-day window using a crossover design. Furthermore, a correlation with a relative adrenal insufficiency was not tested. In this context, the impact of low-dose hydrocortisone application in patients with appropriate adrenal function would be of special interest, possibly elucidating effects beyond restoration of cortisol deficiency. Therefore, further randomized clinical trials with an intent-to-treat design have to be performed to answer these questions.
In conclusion, low-dose hydrocortisone treatment in septic shock rapidly induced hemodynamic stabilization, probably by a reduction of nitric oxide formation. Both inflammatory and antiinflammatory responses were attenuated, whereas the innate cell function of granulocytes and monocytes was preserved. Although hydrocortisone did not aggravate immunosuppression, downregulation of HLA-DR receptors on monocytes has to be considered in severely immunodepressed patients. To avoid rebound effects, low-dose hydrocortisone has to be tapered over days.
We postulate that modulation of the complex immune network in a more widely ranging fashion by the use of low doses of hydrocortisone may be advantageous compared with the inhibition of single mediators, possibly provoking microenvironmental imbalances.
Acknowledgment: The authors thank Margarita Pettersson for her outstanding laboratory skills and technical assistance to the experimental part of the study; the intensive care unit physicians and nurses for supporting the study and excellent patient care; and Gisa Risse, Ph,D., and Mathias Nordman, Ph.D., from the Hospital Pharmacy for blinding, randomization, and preparation of the medication. The authors are grateful to Andrea Berg, Dorit Behnke, and Marko Kayser who participated in data collection and laboratory work. Cortisol was measured by Karl-Heinz Graef (Clinic of Hematology and Oncology, Charite, Campus Virchow Clinic).
References
1. Buckingham JC. Fifteenth Gaddum Memorial Lecture December 1994: stress and the neuroendocrine-immune axis: the pivotal role of glucocorticoids and lipocortin 1. Br J Pharmacol 1996;118:1-19.
2. Meduri GU. An historical review of glucocorticoid treatment in sepsis: disease pathophysiology and the design of treatment investigation. Sepsis 1999;3:21-38.
3. Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med 1995;23:1294-1303.
4. Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 1997;25:1095-- 1100.
5. Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, Duncan RC, Tendler MD, Karpf M. The effects of high-dose corticosteroids in patients with septic shock: a prospective, controlled study. N Engl J Med 1984;311:1137-1143.
6. Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, Hemmer B, Hummel T, Lenhart A, Heyduck M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med 1999;27:723-732.
7. Chawla K, Kupfer Y, Goldman I, Tessler S. Hydrocortisone reverses refractory septic shock [abstract]. Crit Care Med 1999;27:A33.
8. Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med 1998;26:645-650.
9. Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862-871.
10. Briegel J, Schelling G, Haller M, Mraz W, Forst H, Peter K. A comparison of the adrenocortical response during septic shock and after complete recovery. Intensive Care Med 1996;22:894-899.
11. Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet 1991;337:582-583.
12. Sibbald WJ, Short A, Cohen MP, Wilson RF. Variations in adrenocortical responsiveness during severe bacterial infections: unrecognized adrenocortical insufficiency in severe bacteria] infections. Ann Surg 1977; 186:29-33.
13. Soni A, Pepper GM, Wyrwinski PM, Ramirez NE, Simon R, Pina T, Gruenspan H, Vaca CE. Adrenal insufficiency occurring during septic shock: incidence, outcome. and relationship to peripheral cytokine levels. Am J Med 1995;98:266-271.
14. Matot I, Sprung CL. Corticosteroids in septic shock: resurrection of the last rites? Crit Care Med 1998;26:627-629.
15. Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 2000;283:1038-1045.
16. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000;21:55-89.
17. Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med 1996;24:1125-1128.
18. Bone RC. American college of chest physicians/society of critical care medicine consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864-874.
19. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JA MA 1993;270:2957-2963.
20. Vincent JL. Moreno R, Takata J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the Euro
pean Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707-710.
21. Senn S. Cross-over trials in clinical research, 1st ed. New York: John Wiley & Sons; 1993.
22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Star Soc Ser B 1995; 57:289-300.
23. Briegel J, Forst H, Hellinger H, Haller M. Contribution of cortisol deficiency to septic shock. Lancer 1991;338:507-508.
24. Schneider AJ, Voerman HJ. Abrupt hemodynamic improvement in late septic shock with physiological doses of glucocorticoids. Intensive Care Med 1991;17:436-437.
25. Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, Buehler M. Uhl W, Peter K. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome: the Phospholipase A2 Study Group. Clin Investig 1994;72:782-787.
26. Oppert M, Reinicke A, Graf KJ, Barckow D, Frei U, Eckardt KU. Plasma cortisol levels before and during "low-dose" hydrocortisone therapy and their relationship to hemodynamic improvement in patients with septic shock. Intensive Care Med 2000;26:1747-1755.
27. Annane D, Bellissant E, Sebille V, Lesieur 0, Mathieu B, Raphael JC, Gajdos P. Impaired pressor sensitivity to noradrenaline in septic shock patients with and without impaired adrenal function reserve. BrJ Clin Pharmacol 1998;46:589-597.
28. Vincent JL. Zhang H, Szabo C, Preiser JC. Effects of nitric oxide in septic shock. Am J Respir Crit Care Med 2000;161:1781-1785.
29. Kirkeboen KA, Strand OA. The role of nitric oxide in sepsis: an overview. Acta Anaesthesiol Scand 1999;43:275 288.
30. Parker MM. Pathophysiology of cardiovascular dysfunction in septic shock. New Horiz 1998;6:130-138.
31. Endo S, Inada K, Nakae H, Arakawa N, Takakuwa T, Yamada Y, Shimamura T, Suzuki T, Taniguchi S, Yoshida M. Nitrite/nitrate oxide (NOx) and cytokine levels in patients with septic shock. Res Commun Mol Pathol Pharmacol 1996;91:347-356.
32. Groeneveld PH, Kwappenberg KM, Langermans JA, Nibbering PH, Curtis L. Nitric oxide (NO) production correlates with renal insufficiency and multiple organ dysfunction syndrome in severe sepsis. Intensive Care Med 1996;22:1197-1202.
33. Arnalich F, Hernanz A, Jimenez M, Lopez J, Tato E, Vazquez JJ, Montiel C. Relationship between circulating levels of calcitonin gene-related peptide, nitric oxide metabolites and hemodynamic changes in human septic shock. Regul Pept 1996;65:115-121.
34. Doughty LA, Carcillo JA, Kaplan S, Janosky J. Plasma nitrite and nitrate concentrations and multiple organ failure in pediatric sepsis. Crit Care Med 1998;26:157-162.
35. Vallance P, Rees D, Moncada S. Therapeutic potential of NOS inhibitors in septic shock. In: Mayer B, editor. Nitric oxide. Berlin: Springer Verlag; 2000. p. 385-397.
36. Grover R, Lopez A, Lorente J, Steingrub J, Bakker J, Wilatts S. Multicenter, randomized, placebo-controlled, double-blind study of the nitric oxide inhibitor 546V88: effect on survival in patients with septic shock [abstract]. Crit Care Med 1999;27:A33.
37. Kilbourn RG, Szabo C, Traber DL. Beneficial versus detrimental effects of nitric oxide synthase inhibitors in circulatory shock: lessons learned from experimental and clinical studies. Shock 1997;7:235-246.
38. Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA 1990: 87:10043-10047.
39. Salyapongse AN, Billiar TR. Nitric oxide as a modulator of sepsis: therapeutic possibilities. In: Baue AE, Faist E, Fry DE, editors. Multiple organ failure: pathophysiology, prevention, and therapy. New York: Springer Verlag; 2000. p. 176-187.
40. Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA 1990; 87:10043-10047.
41. Simmons WW, Ungureanu-Longrois D, Smith GK, Smith TW, Kelly RA. Glucocorticoids regulate inducible nitric oxide synthase by inhibiting tetrahydrobiopterin synthesis and L-arginine transport. J Biol Chem 1996:271:23928-23937.
42. Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells: evidence for the involvement of transcriptional and posttranscriptional regulation. Proc Natl Acad Sci USA 1996;93:255-259.
43. Walker G. Pfeilschifter J, Kunz D. Mechanisms of suppression of induc
ible nitric-oxide synthase (iNOS) expression in interferon (IFN)gamma-stimulated RAW 264.7 cells by dexamethasone: evidence for glucocorticoid-induced degradation of iNOS protein by calpain as a key step in post-transcriptional regulation. . Biol Chem 1997:272: 16679-16687.
44. Walker BR, Williams BC. Corticosteroids and vascular tone: mapping the messenger maze. Clin Sci (Load) 1992;82:597-605.
45. Ullian ME. The role of corticosteroids in the regulation of vascular tone. Cardiovasc Res 1999;41:55-64.
46. Damas P. Canivet JL, De Groote D, Vrindts Y, Albert A, Franchimont P, Lamy M. Sepsis and serum cytokine concentrations. Crit Care Med 1997;25:405-412.
47. Fujishima S, Sasaki J, Shinozawa Y, Takuma K, Kimura H, Suzuki M, Kanazawa M. Hori S, Aikawa N. Serum MIP-1 alpha and IL-8 in septic patients. Intensive Care Med 1996;22:1169-1175.
48. Marty C, Misset B, Tamion F, Fitting C, Carlet J, Cavaillon JM. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med 1994;22:673-679.
49. Calandra T, Gerain J, Heumann D, Baumgartner JD, Glauser MP. High circulating levels of interleukin-6 in patients with septic shock: evolution during sepsis, prognostic value, and interplay with other cytokines: the Swiss-Dutch JS Immunoglobulin Study Group. An J Med 1991; 91:23-29.
50. Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, Franchimont P, Lamy M. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 1992:215:356-362.
51. Adams DH, Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet 1994;343:831-836.
52. Cowley HC, Heney D, Gearing AJ, Hemingway I, Webster NR. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: a prospective cohort study. Crit Care Med 1994;22:651-657.
53. Endo S, Inada K, Kasai T, Takakuwa T, Yamada Y, Koike S, Wakabayashi G, Niimi M, Taniguchi S, Yoshida M. Levels of soluble adhesion molecules and cytokines in patients with septic multiple organ failure. J Inflamm 1995;46:212-219.
54. Muller Kobold AC, Tulleken JE, Zijlstra JG. Sluiter W, Hermans J, Kallenberg CG, Cohen Tervaert JW. Leukocyte activation in sepsis: correlations with disease state and mortality. Intensive Care Med 2000; 26:883-892.
55. Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock: studies of HLA-DR, CDllb, CD14, and IL-2R expression. Chest 1993;104:847-853.
56. Nakagawa M, Bondy GP, Waisman D, Minshall D, Hogg JC, van Eeden SF. The effect of glucocorticoids on the expression of L-selectin on polymorphonuclear leukocyte. Blood 1999;93:2730-2737.
57. Filep JG, Delalandre A, Payette Y, Folder-Filep E. Glucocorticoid receptor regulates expression of L-selectin and CD I 1/CD18 on human neutrophils. Circulation 1997;96:295-301.
58. McGill SN, Ahmed NA, Hu F, Michel RP, Christou NV. Shedding of L-selectin as a mechanism for reduced polymorphonuclear neutrophil exudation in patients with the systemic inflammatory response syndrome. Arch Surg 1996;131:1141-1147.
59. Briegel J, Jochum M, Gippner-Steppert C, Thiel M. Immunomodulation in septic shock: hydrocortisone differentially regulates cytokine responses. J Am Soc Nephrol 2001;12:S70-574.
60. van der Poll T, van Deventer SJH. Interleukin-6 in bacterial infection and sepsis: innocent bystander or essential mediator? In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. New York: Springer Verlag; 1999. p. 43-53.
61. Mosmann TR, Sad S. The expanding universe of T-cell subsets: Thl, Th2 and more. Immunol Today 1996;17:139 146.
62. Almawi WY, Melemedjian OK, Rieder MJ. An alternate mechanism of glucocorticoid anti-proliferative effect: promotion of a Th2 cytokine-- secreting profile. Clin Transplant 1999;13:365-374.
63. Ramirez F, Fowell DJ, Puklavec M, Simmonds S. Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol 1996;156:2406-2412.
64. Zedler S, Bone RC, Baue AE, Von Donnersmarck GH, Faist E. T-cell reactivity and its predictive role in immunosuppression after burns. Crit Care Med 1999;27:66-72.
65. Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians 1996;108:374-381.
66. Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, Frucht DM, Chrousos GP, O'Shea JJ. Inhibition of Thl immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-- induced Stat4 phosphorylation in T lymphocytes. J Immunol 2000;164: 1768-1774.
67. Larsson S, Linden M. Effects of a corticosteroid, budesonide, on production of bioactive IL-12 by human monocytes. Cytokine 1998;10: 786-789.
68. Wilckens T, Dc Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today 1997:18:418-424.
69. Hershman MJ, Cheadle WG, Kuftinec D, Polk HC Jr, George CD. An outcome predictive score for sepsis and death following injury. Injury 1988:19:263-266.
70. Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, Docke WD, Kox WJ. Monocyte deactivation-rationale for a new therapeutic strategy in sepsis. Intensive Care Med 1996;22:474-481.
71. Tschaikowsky K, Hedwig-Geissing M, Schiele A, Bremer F, Schywalsky M, Schuttler J. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-- reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med 2002;30:1015-1023.
72. Satoh A, Miura T, Satoh K, Masamune A, Yamagiwa T, Sakai Y, Shibuya K, Takeda K. Kaku M, Shimosegawa T. Human leukocyte antigen-- DR expression on peripheral monocytes as a predictive marker of sepsis during acute pancreatitis. Pancreas 2002;25:245-250.
73. Kox WJ, Bone RC, Krausch D, Docke WD, Kox SN, Wauer H, Egerer K, Querner S, Asadullah K, Von Baehr R, et al. Interferon gamma-lb in the treatment of compensatory anti-inflammatory response syndrome: a new approach: proof of principle. Arch Intern Med 1997;157: 389-393.
Didier Keh, Thomas Boehnke, Steffen Weber-Cartens, Christina Schulz, Olaf Ahlers, Sven Bercker, Hans-Dieter Volk, Wolf-Dietrich Doecke, Konrad 1. Falke, and Herwig Gerlach
Department of Anesthesiology and Intensive Care Medicine; Institute of Medical Immunology, Charite, Humboldt University; and Vivantes Klinikum Neukoelln, Berlin, Germany
(Received in original form May 19, 2002; accepted in final form October 31, 2002)
Supported by Deutsche Forschungsgemeinschaft (DFG Fa: 139/20 (expenses for analytes) and Pharmacia/Upjohn (expenses for the patients' insurance)
Correspondence and requests for reprints should be addressed to Didier Keh, M.D., Department of Anesthesiology and Intensive Care Medicine, Charite-Campus Virchow Clinic, Humboldt University, Augustenburger Platz 1, D-1 3353 Berlin, Germany. E-mail: didier.keh@charite.de
Copyright American Thoracic Society Feb 15, 2003
Provided by ProQuest Information and Learning Company. All rights Reserved