METHOD OF PREPARATION
Note: This preparation should be prepared in a laminar airflow hood in a cleanroom (or via isolation barrier technology) by a validated aseptic compounding pharmacist using strict aseptic technique.
1. Calculate the required quantity of each ingredient for the total amount to be prepared.
2. Accurately weigh and/or measure each ingredient.
3. Dissolve the scopolamine hydrobromide, citric acid, dibasic sodium phosphate and sodium chloride in approximately 95 mL of sterile water for injection.
4. Add the benzalkonium chloride 50% solution and mix well.
5. Adjust the pH if necessary, using either additional citric acid or sodium hydroxide 10% aqueous solution to a pH of 6.5.
6. Add sufficient sterile water for injection to volume and mix well.
7. Filter through an appropriate sicrilc 0.2-µm filter into a sterile container.
8. Package and label.
PACKAGING
Package in nasal-spray containers that are tight and light-resistant.1
LABELING
Keep out of reach of children. Use only as directed. For the nose.
STABILITY
A beyond-use date of up to 6 months can be used for this preparation.1
USE
Scopolamine hydrobromide 0.2% nasal spray has been used in the treatment of motion sickness.2
QUALITY CONTROL
Quality-control assessment can include final volume, pH, specific gravity, active-drug assay, color, clarity, physical observation, physical stability (discoloration, foreign materials, gas formation, mold growth), osmolality and sterility.3-5
DISCUSSION
Scopolamine hydrobromide is a naturally occurring tertiary amine antimuscarinic used for the prevention of nausea and vomiting induced by motion. An advantage to this nasal formulation is a rapid onset of action.
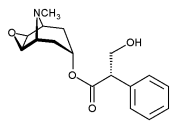
Scopolamine hydrobromide (C^sub 17^H^sub 21^NO^sub 4^.HBr.^sub 3^H2O, MW 438.31, hyoscine hydrobromide) occurs as colorless or white crystals or as a white, granular powder. It melts at about 197°C with decomposition. It is odorless and slightly efflorescent in dry air. It is freely soluble in water (l g in 1.5 mL) and soluble in alcohol (l g in 20 mL).1,6
The pH of the solution has been shown to affect the extent and rate of absorption. In a study using solutions prepared at pH levels of 4.0, 7.0 and 9.0, the higher the pH, the faster were the T^sub max^ values and the higher were the C^sub max^ values. The pH 7.0 formulation selected here provides relatively rapid onset of action and good absorption and is in the allowable pH range of the USP injection that goes up to pH 6.5.1,7
Citric acid, available in an anhydrous form (C^sub 6^H^sub 8^O^sub 7^, MW 192.12) or in a monohydrate form (C^sub 6^H^sub 8^O^sub 7^.H2O, MW 210.14), occurs as colorless or translucent crystals, or as a white crystalline. One gram is soluble in less than 1 mL of water and 1.5 mL of ethanol.8
Dibasic sodium phosphate (NaHPO^sub 4^.xH2O) is available in an anhydrous form (MW 141.96) and as a dihydrate (MW 177.99), heptahydrate (MW 268.07) and dodecahydrate (MW 358.14). It is used as a buffering agent and as a sequestering agent. It is very soluble in water but practically insoluble in ethanol.9
Benzalkonium chloride is a bactericidal antimicrobial. Benzalkonium Chloride Solution NF is a clear liquid, colorless or slightly yellow unless a color has been added. Benzalkonium chloride is composed of a mixture of straight-chain homologs.10
REFERENCES
1. US Pharmacopeial Convention, Inc. United States Pharmacopeia 26-National Formulary 21. Rockville, MD:US Pharmacopeial Convention, Inc.; 2003:1668,1939, 2197-2201, 2580.
2. Klocker N, Hanschke W, Toussaint S et al. Scopolamine nasal spray in mot!on sickness: A randomized, controlled, and crossover study for the comparison of two scopolamine nasal sprays with oral dimenhydrinate and placebo. Eur J Pharm Sci 2001;13:227-232.
3. Alien LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC 1999;3:146-147.
4. Alien LV Jr. Standard operating procedure for particulate testing for sterile products. IJPC1998;2:78.
5. Alien LV Jr. Standard operating procedure: Quality assessment for injectable solutions. IJPC 1999;3:406-407.
6. McEvoy GK, ed. XlWFS Drug Information-2003. Bethesda, PVID:American Society of Health-System Pharmacists; 2003:1214-1218.
7. Ahmed S, Sileno AP, deMeireles JC et al. Effects of pH and dose on nasal absorption of scopolamine hydrobromide in human subjects. Pharm Res 2000;17:974-977.
8. Amidon GE. Citric acid monohydrate. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:140-142.
9. Kearney AS. Sodium phosphate, dibasic. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:493-495.
10. Kibbe AH. Benzalkonium chloride. In: Kibbe AH, ed. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC:American Pharmaceutical Association; 2000:33-35.
Copyright International Journal of Pharmaceutical Compounding Jan/Feb 2004
Provided by ProQuest Information and Learning Company. All rights Reserved