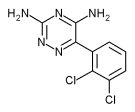
The U.S. Food and Drug Administration has approved lamotrigine (Lamictal) for the long-term maintenance treatment of adults with bipolar I disorder to delay the time to occurrence of mood episodes (depression, mania, hypomania, mixed episodes) in patients treated for acute mood episodes with standard therapy.
The FDA has noted that the findings for lamotrigine maintenance treatment were more robust in bipolar depression. The effectiveness of lamotrigine in the acute treatment of mood episodes has not been established.
According to the manufacturer, the most common side effects are nausea, insomnia, somnolence, back pain, fatigue, rhinitis, nonserious rash, abdominal pain, dry mouth, constipation, vomiting, exacerbation of cough, and pharyngitis. Serious rashes requiring hospitalization and discontinuation of therapy, such as Stevens-Johnson syndrome, have been reported in association with the use of lamotrigine. The safety and effectiveness of lamotrigine have not been established as initial monotherapy, for conversion to monotherapy from nonenzyme-inducing antiepileptic drugs, or for simultaneous conversion to monotherapy from two or more concomitant antiepileptic drugs.
Lamotrigine has been available since 1994 and is indicated as adjunctive therapy for partial seizures in adults and children.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group