Abstract
Laryngomalacia is the most common cause of stridor in newborns and infants. Patients usually present with an inspiratory stridor only, although some exhibit other anomalies. To rule out other possible pathologies, bronchoscopy is advisable. However, the authors of some recent studies have advocated the use of fiberoptic laryngoscopy as a more cost-effective and less-invasive alternative. No surgical intervention is required to treat laryngomalacia in most cases. The disease usually resolves spontaneously by the time a patient reaches the age of 24 months. In this article, we describe a case of laryngomalacia that was atypical in that the patient was 10 years old. We also review the literature in an effort to increase awareness of this condition.
Introduction
Laryngomalacia is the most common congenital malformation of the larynx, and it is the most common cause of stridor in newborns and infants. (1) It manifests as an inspiratory stridor and is usually characterized by a highpitched, fluttering voice. (2) Although affected patients do not exhibit much in the way of other physical symptoms, the unusual voice is worrisome to their parents. The stridor usually worsens during the first 8 months of life, reaches a plateau at 9 to 12 months, and generally resolves gradually by the rime the patient reaches 24 months of age. (3)
No intervention is necessary for most patients. Surgery is required only when there are signs of failure to thrive, obstructive sleep apnea, cor pulmonale, severe reflux, or apnea while awake or when the disease does not spontaneously resolve as anticipated. Persistence beyond 24 months is rare, but a few case reports have been documented, some of which occurred as late as adolescence. (4,5)
In this article, we describe an unusual presentation of laryngomalacia, and we review the literature in an attempt to increase physician awareness of this disease.
Case report
A 10-year-old boy was brought to the ENT clinic at The Aga Khan University Hospital (AKUH) in Karachi with a history of a continuous harsh voice since birth. He was noted to have inspiratory stridor that became worse on exertion and when he bent forward. No other aggravating or relieving factors were noted. The patient's stridor had first been evaluated by a general practitioner when the boy was 1 month old. The physician prescribed amoxicillin syrup and advised the boy's parents that the stridor would persist until the boy reached 2 years of age. The antibiotic syrup failed to improve the patient's condition.
When the patient was 3 years old, he was taken to a local hospital, where he was given a provisional diagnosis of laryngomalacia and prescribed terbutaline syrup. Physicians there suggested that he undergo direct bronchoscopy, and they referred him to AKUH. However, the parents could not afford to pay for the bronchoscopy, and they did not follow up on the recommendation. The boy did show some improvement with the terbutaline.
During our evaluation, we learned that the patient had been born at term and at home; the delivery was uncomplicated. Although his birth weight and other anthropometric parameters at the time of his birth were not known, he appeared to be healthy. His mother had not been exposed to medicines or teratogens during her pregnancy. The boy's parents were healthy and not consanguineous. He grew normally and achieved appropriate developmental milestones. He began speaking one-syllable letters at 12 months of age and brief sentences at 2 years. He was sociable and exhibited no behavioral problems.
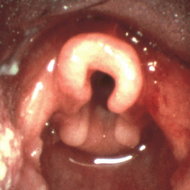
This time, bronchoscopy was performed at AKUH, and it revealed that the patient had an omega-shaped epiglottis but no other obvious physical abnormality in the airway. However, during inspiration, the airway was seen to collapse, and flabby aryepiglottic folds were sucked in. Based on these findings, the diagnosis of laryngomalacia was confirmed.
The patient was discharged with an assurance that his condition was self-limited and would resolve in time. No change in his symptoms was noted on the first follow-up 1 week later. One year later, the frequency of his inspiratory stridor had markedly decreased, and it occurred only on strenuous exertion. Otherwise, the child was living a normal life.
Discussion
Congenital stridor was reported as early as 1853 by two French physicians, Rilliet and Barthez. (6) They described a newborn who had both inspiratory and expiratory stridor; the child was otherwise normal. The first comprehensive review of this condition was published by Sutherland and Lack in 1897. (7) In their article, entitled "Congenital laryngeal obstruction," they described a series of 18 patients with laryngomalacia, which they called congenital laryngeal stridor. In 1942, Jackson was the first to use the term laryngomalacia (from the Greek malakia: morbid softening of part of an organ); he defined it as a softness, flabbiness, or loss of consistency of laryngeal tissues. (8)
In affected patients, inspiratory stridor is usually present at birth, although in some cases it does not become apparent until weeks or months later. In some infants, the stridor does not manifest until the child becomes more active at approximately 3 months of age; in other cases, the stridor is precipitated by an upper respiratory infection. (9) Sometimes vibrations can be felt by placing a hand on the infant's chest. Parents have described the stridor as purring, crackling, crowing, croaking, and squeaking. Affected infants are neither dyspneic nor generally uncomfortable. However, in 10% of cases, the upper airway obstruction is severe enough to cause apnea or failure to thrive, which necessitates surgical intervention. (10)
The stridor is often intermittent; it is exacerbated when the child is active and crying. (4,7) It can be aggravated by upper respiratory tract infections, which increase the risk of aspiration. The severity of the stridor can be affected by body position--that is, it is aggravated by supination and head flexion and is relieved by pronation and head extension. (7,11) Some cases have been reported in which the condition was severe enough to cause intercostal, abdominal, supraclavicular, infraclavicular, and xiphoid retractions, which can lead to pectus excavatum. (12,13) Cyanosis is rare. (9,14) Reports of stridor during sleep are variable. (4,9, 11)
Etiology. The etiology of laryngomalacia remains unknown. Four primary causes have been theorized over the years: (1) cartilage immaturity, (2) an anatomic abnormality, (3) neuromuscular immaturity, and (4) the presence of gastroesophageal reflux disease (GERD):
Cartilage immaturity. In the late 19th century, Sutherland and Lack proposed that laryngomalacia is related to a delay in the normal development of cartilaginous support of the arytenoids and epiglottic tissues. (7) Since then, this theory has been disproved. Chandra et al showed that laryngomalacia does not represent a histologically identifiable chondropathy and that cartilaginous abnormalities play no role in its pathogenesis. (15) Moreover, the literature reveals that infants even 2 or 3 months immature are no more likely to experience laryngomalacia than are term infants.
Anatomic abnormality. In 1922, Iglauer proposed that laryngomalacia is the result of an exaggeration of the infantile larynx. (12) The laryngeal tissues of infants are relatively soft and flaccid and their aryepiglottic folds and arytenoid tissues are relatively large; as a result, their glottic opening is smaller and their larynx is flabbier and softer. The elongated, tubular epiglottis and closely approximated aryepiglottic folds are drawn inward on inspiration because the supporting framework is too pliable. The folded epiglottis acquires an "omega" or tubular shape. As the child grows older, the tissues become resistant to inspiratory forces. This theory is supported by several findings that have led endoscopists to describe the larynx of children with laryngomalacia as infantile. (16) But omega- and tube-shaped epiglottises exist in nonstridorous infants as well, indicating that they are not an important factor in causing laryngomalacia.
Neuromuscular immaturity. There is a high prevalence of neurologic disorders in patients with laryngomalacia, which has led some investigators to believe that laryngomalacia is caused by neuromuscular immaturity and consequent laryngeal hypotonia. However, this theory needs further investigation. (16,17)
GERD. At one time, it was believed that GERD occurred in 35 to 68% of infants with laryngomalacia. (18,19) However, in 1999, Matthews et al, using double-probe pH testing, reported that GERD was present in almost 100% of cases of laryngomalacia. (20) Despite attempts to identify the mechanism, no link between GERD and stridor has been confirmed. It is not certain whether GERD causes airway symptoms or vice versa. Orenstein and Orenstein proposed that reflux of gastric contents causes supraglottic edema and changes airway resistance, thereby causing airway obstruction. (21) On the other hand, Wang et al speculated that the
increased negative intrathoracic pressure during inspiration in an obstructed laryngeal airway could predispose a patient to GERD by trying to overcome the lower esophageal sphincter. (22) Nevertheless, surgical treatment of GERD often fails to relieve respiratory symptoms. (23) Hadfield et al recently showed that treating laryngomalacia by aryepiglottoplasty alleviated GERD, suggesting that laryngomalacia plays a causative role in GERD. (24)
Various mechanisms have been proposed as the cause of airway obstruction, but six deserve mention. (25) They include (1) the inward collapse of the aryepiglottic folds, primarily the cuneiform cartilages, (2) an elongated epiglottis curled on an abnormality, (3) anterior and medial collapsing movements of the arytenoid cartilages, (4) posterior and inferior displacement of the epiglottis, (5) short aryepiglottic folds, and (6) an overly acute angle of the epiglottis.
A possible familial predisposition was first noted in 1953 by Apley, who found stridor in 15 children from five families. (26) Later, Kahn et al found a positive family history in 3 patients. (27) Shulman et al described a family in which 3 of 5 siblings had laryngomalacia. (28) Shohat et al described an autosomal-dominant pattern of inheritance in a family in which 9 members of three generations were affected. (29) Harreus and Issing reported the case of an 8-month-old girl who presented with both laryngomalacia and a combined hearing loss; in this case, there was a deletion of the long arm of chromosome 5q. (30) E1-Shanti et al found a new syndrome in 5 patients from two separate families who had laryngomalacia, XY gonadal dysgenesis, and alopecia universalis congenita. (31) However, the results of other studies (9,32) have not supported a familial relationship, and no genetic studies of laryngomalacia have been reported.
Endoscopic investigations. Laryngomalacia occurs both in isolation and in association with other anomalies of the airway or other organ systems. Synchronous airway lesions have been reported in 19% of affected infants. (33) Some investigators believe that the presence of other secondary airway lesions in all infants with laryngomalacia mandates direct laryngoscopy and bronchoscopy in order to avoid the risk of missing a potentially life-threatening secondary airway lesion. (34) Because these procedures require general anesthesia, the risk of associated morbidity and mortality must be considered. Many investigators believe that direct bronchoscopy should not be performed on all infants in light of not only anesthesia-related complications, but cost as well. (35) In fact, reports indicate that intervention for these secondary lesions was required in only 4.7% infants with laryngomalacia. (34,36)
According to Botma et al, a better alternative as a firstline investigation in children with stridor is fiberoptic laryngoscopy; they recommend that bronchoscopy be reserved for those patients who are likely to have a pathology other than laryngomalacia and for those who fail to thrive. (35) Fiberoptic laryngoscopy is safe and costeffective; in fact, it is 100 times less expensive than direct bronchoscopy. Fiberoptic laryngoscopy was first used to investigate stridor in infants by Silberman et al in the 1970s. (37) In 2000, Botma et al reported the results of their investigation of the role of fiberoptic laryngoscopy in 43 infants with stridor. (35) Of this population, 35 were diagnosed with laryngomalacia, 6 had vocal fold palsies, and 2 were normal. These findings indicate that fiberoptic laryngoscopy is fairly sensitive in diagnosing laryngomalacia.
Surgery. Surgical intervention is not required for most patients in whom laryngomalacia is self-limited because most mature normally. If the child's general progress is satisfactory, active management is not necessary, and parents can be reassured that the symptoms will subside over time. Surgery is required only in severe cases--those that involve the collapse of the glottis on inspiration and the presence of complications of obstruction (e.g., failure to thrive, obstructive sleep apnea, cor pulmonale, severe reflux, or apnea while awake).
Iglauer was first to perform surgery on a patient with laryngomalacia (he amputated the epiglottis with a wire snare), a case that he reported in 1922. (12) Endoscopic surgery was first reported in 1984 by Lane et al, who noted that it led to a dramatic improvement in a 3-month-old patient; they excised the lateral portions of the epiglottis, the corniculate cartilage, and the tips of the arytenoids. (3)
Use of a C[O.sub.2] laser was first reported by Seid et al in 1985; they divided the aryepiglottic fold with the laser in 2 patients. (38) Since then, various surgical modifications have been developed. Surgeries today are usually performed with a C[O.sub.2] laser and microdissection. The procedures that have been advocated include (1) division of the aryepiglottic fold, (2) partial amputation of the epiglottis, (3) suture of the epiglottis to the base of the tongue (epiglottoplasty), (4) removal of redundant supra-arytenoid mucosa and the lateral borders of the epiglottis, and (5) removal of the cuneiform and corniculate cartilages and the surrounding mucosa. In extreme cases, tracheostomy is performed.
Kelly and Gray (39) and Reddy and Matt (40) reviewed the complications of surgical procedures for laryngomalacia and compared the outcomes of patients who underwent unilateral and bilateral supraglottoplasty. They recommended the unilateral procedure as the first-line surgical treatment of severe laryngomalacia that requires surgery.
Acknowledgments
The authors thank Shaikh Rahmatullah, a secretary in the Otolaryngology--Head and Neck Surgery unit at The Aga Khan University, for his cooperation and clerical help in the preparation of the manuscript.
References
(1.) Rowe LD. Airway obstruction in the pediatric patient. Prim Care 1982;9:317-36.
(2.) Holinger PH, Johnston KC. The infant with respiratory stridor. Pediatr Clin North Am 1955;6:403-11.
(3.) Lane RW, Welder DJ, Steinem C, Marin-Padilla M. Laryngomalacia. A review and case report of surgical treatment with resolution of pectus excavatum. Arch Otolaryngol 1984;110: 546-51.
(4.) Cinnamond MJ. Congenital disorders of the larynx, trachea and bronchi. In: Kerr AG, ed. Scott-Brown's Otolaryngology. 5th ed., vol. 6. Boston: Butterworths 1987:412-13.
(5.) Gessler EM, Simko EJ, Greinwald JH, Jr. Adult laryngomalacia: An uncommon clinical entity. Am J Otolaryngol 2002;23:386-9.
(6.) Rilliet F, Barthez E. Traite Clinique et Pratique des Maladies des Enfants. Vol 1. Paris: Germer Bailliere, 1853:484-8.
(7.) Sutherland GA, Lack HL. Congenital laryngeal obstruction. Lancet 1897;2:653-5.
(8.) Jackson C. Diseases and Injuries of the Larynx. New York: MacMillan, 1942:63-8.
(9.) McSwiney PF, Cavanagh NP, Languth P. Outcome in congenital stridor (laryngomalacia). Arch Dis Child 1977;52:215-18.
(10.) Kavanagh KT, Babin RW. Endoscopic surgical management for laryngomalacia. Case report and review of the literature. Ann Otol Rhinol Laryngol 1987;96:650-3.
(11.) Ferguson CF. Congenital abnormalities of the infant larynx. Otolaryngol Clin North Am 1970;3:185-200.
(12.) Iglauer S. Epiglottidectomy for the relief of congenital laryngeal stridor with report of a case. Laryngoscope 1922;32:56-9.
(13.) Atkins JP. Laryngeal problems of infancy and childhood. Pediatr Clin North Am 1962;9:1125-35.
(14.) Schwartz L. Congenital laryngeal stridor (inspiratory laryngeal collapse): A new theory as to its underlying cause and the desirability of a change in terminology. Arch Otolaryngol 1944; 39:403-12.
(15.) Chandra RK, Gerber ME, Holinger LD. Histological insight into the pathogenesis of severe laryngomalacia. Int J Pediatr Otorhinolaryngol 2001;61:31-8.
(16.) Belmont JR, Grundfast K. Congenital laryngeal stridor (laryngomalacia): Etiologic factors and associated disorders. Ann Otol Rhinol Laryngol 1984;93:430-7.
(17.) Archer SM. Acquired flaccid larynx. A case report supporting the neurologic theory of laryngomalacia. Arch Otolaryngol Head Neck Surg 1992;118:654-7.
(18.) Nussbaum E, Maggi JC. Laryngomalacia in children. Chest 1990;98:942-4.
(19.) Roger G. Denoyelle F, Triglia JM, Garabedian EN. Severe laryngomalacia: Surgical indications and results in 115 patients. Laryngoscope 1995;105:1111-17.
(20.) Matthews BL, Little JP, McGuirt WF, Jr., Koufman JA. Reflux in infants with laryngomalacia: Results of 24-hour double-probe pH monitoring. Otolaryngol Head Neck Surg 1999;120:860-4.
(21.) Orenstein SR, Orenstein DM. Gastroesophageal reflux and respiratory disease in children. J Pediatr 1988;122:847-58.
(22.) Wang W, Tovar JA, Eizaguirre I, Aldazabal P. [Airway obstruction associated with gastroesophageal reflux: Experimental study]. Cir Pediatr 1993;6:76-8.
(23.) Eizaguirre I, Tovar JA. Predicting preoperatively the outcome of respiratory symptoms of gastroesophageal reflux. J Pediatr Surg 1992;27:848-51.
(24.) Hadfield PJ, Albert DM, Bailey CM, et al. The effect of aryepiglottoplasty for laryngomalacia on gastro-oesophageal reflux. Int J Pediatr Otorhinolaryngol 2003;67:11-14.
(25.) Chen JC. Holinger LD. Congenital laryngeal lesions: Pathology study using serial macrosections and review of the literature. Pediatr Pathol 1994;14:301-25.
(26.) Apley J. The infant with stridor: A follow-up survey of 80 cases. Arch Dis Child 1953;28:423-35.
(27.) Kahn A, Baran D, Spehl M, et al. Congenital stridor in infancy. Clinical lessons derived from a survey of 31 instances. Clin Pediatr (Phila) 1977:16: 19-26.
(28.) Shulman JB, Hollister DW, Thibeault DW, Krugman ME. Familial laryngomalacia: A case report. Laryngoscope 1976;86:84-91.
(29.) Shohat M, Sivan Y, Taub E, Davidson S. Autosomal dominant congenital laryngomalacia. Am J Med Genet 1992;42:813-14.
(30.) Harreus UA, Issing WJ. [Chromosome 5q-syndrome-ENT pathologies]. Laryngorhinootologie 2002;81:565-7.
(31.) El-Shanti H, Ahmad M, Ajlouni K. Alopecia universalis congenita, XY gonadal dysgenesis and laryngomalacia: A novel malformation syndrome. Eur J Pediatr 2003;162:36-40.
(32.) Phelan PD, Gillam GL, Stocks JG, Williams HE. The clinical and physiological manifestations of the "infantile" larynx: Natural history and relationship to mental retardation. Aust Paediatr J 1971;7:135-40.
(33.) Bluestone CD, Healy GB, Cotton RT. Diagnosis of laryngomalacia is not enough! Arch Otolaryngol Head Neck Surg 1996;122: 1417-18.
(34.) Mancuso RF, Choi SS, Zalzal GH, Grundfast KM. Laryngomalacia. The search for the second lesion. Arch Otolaryngol Head Neck Surg 1996;122:302-6.
(35.) Botma M, Kishore A, Kubba H, Geddes N. The role of fibreoptic laryngoscopy in infants with stridor. Int J Pediatr Otorhinolaryngol 2000;55:17-20.
(36.) Gonzalez C, Reilly JS, Bluestone CD. Synchronous airway lesions in infancy. Ann Otol Rhinol Laryngol 1987;96:77-80.
(37.) Silberman HD, Wilf H, Tucker JA. Flexible fiberoptic nasopharyngolaryngoscopy. Ann Otol Rhinol Laryngol 1976;85:640-5.
(38.) Seid AB, Park SM, Kearns MJ, Gugenheim S. Laser division of the aryepiglottic folds for severe laryngomalacia. Int J Pediatr Otorhinolaryngol 1985;10:153-8.
(39.) Kelly SM, Gray SD. Unilateral endoscopic supraglottoplasty for severe laryngomalacia. Arch Otolaryngol Head Neck Surg 1995; 121:1351-4.
(40.) Reddy DK, Matt BH. Unilateral vs. bilateral supraglottoplasty for severe laryngomalacia in children. Arch Otolaryngol Head Neck Surg 2001; 127:694-9.
From the Department of Surgery (Dr. Awan) and the Department of Biological and Biomedical Sciences (Mr. Saleheen), The Aga Khan University, Karachi, Pakistan; and the Department of ENT, the Fauji Foundation Hospital, Rawalpindi, Pakistan (Dr. Ahmad).
Reprint requests: Dr. Sohail Awan, Otolaryngology--Head and Neck Surgery, Department of Surgery, The Aga Khan University Hospital, Stadium Rd., Karachi 74800, Pakistan. Phone: 92-21-48594769; fax: 92-21-493-4294 or 92-21-493-2095; e-mail: sohail_awan50@hotmail.com
COPYRIGHT 2004 Medquest Communications, LLC
COPYRIGHT 2004 Gale Group