Business Editors/Health/Medical Writers
LIONVILLE, Pa. and MANHASSETT, N.Y.--(BUSINESS WIRE)--May 29, 2003
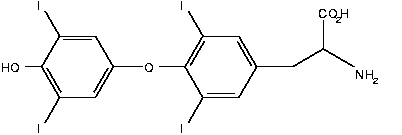
West Pharmaceutical Services, Inc. (NYSE: WST) and T3 Therapeutics LLC today announced that they have signed a worldwide licensing agreement for the development and commercialization of an oral sustained release formulation of liothyronine (T3), used for the treatment of congestive heart failure and hypothyroidism.
Liothyronine is a naturally occurring hormone produced by the thyroid gland that is deficient in patients with congestive heart failure and with hypothyroidism, where it often causes cardiovascular disease. Currently, liothyronine is taken orally by patients suffering from hypothyroidism. According to the American Thyroid Association, hypothyroidism can lead to the buildup of cholesterol in the vascular system and can cause plaque (hardening of the arteries), which can increase the risk of heart attack.
"Our agreement with West Pharmaceutical Services marks an important milestone in realizing our goal to develop formulations of liothyronine (T3) for the treatment of cardiovascular and endocrine diseases," said Dr. Irwin Klein, President of T3 Therapeutics LLC.
Despite their popularity, traditional oral delivery systems are not without problems. For example, the oral delivery of some drugs is associated with relatively poor drug bioavailability and highly variable blood drug levels, thereby requiring multiple dosing, which can compromise cost effectiveness and patient compliance. West's innovative formulation technologies are used to regulate the oral delivery of pharmaceuticals, allowing for the controlled release of the drug into the blood stream. In the case of hypothyroidism, the precise amount of liothyronine released into the bloodstream has a significant effect on the patient's therapeutic benefit.
According to Dr. Bruce Morra, President of West's Drug Delivery Systems Division, "Our collaboration with T3 Therapeutics builds on West's considerable expertise in formulation development of dosage forms and complements our solid base in nasal delivery technologies, bioanalytical evaluation and analytical development. Although it is in its early stages, we believe this program, if successful, can lead to improved therapies for millions of patients worldwide."
About West Pharmaceutical Services Drug Delivery Systems Division
West Pharmaceutical Services Drug Delivery Systems Division provides clients with the science, engineering and know-how necessary to take pharmaceutical products to the market. West's innovative product development has led to significant proprietary technologies in nasal formulations for systemic or local delivery, advanced oral delivery systems, transmucosal vaccine delivery and parenteral drug delivery.
Additionally, West supports its clients in all aspects of pharmaceutical development, from initial feasibility studies to formulation optimization and product development. For more information visit www.westdrugdelivery.com.
About T3 Therapeutics Corporation
T3 Therapeutics LLC is an early stage biopharmaceutical company dedicated to the development of thyroid hormone based therapies for human disease states. T3 Therapeutics has identified the potential for and currently holds a U.S. patent for T3 based therapies for heart failure and for the over 20 million patients with hypothyroidism currently being treated with T4 alone.
About West Pharmaceutical Services
West Pharmaceutical Services, Inc. (NYSE: WST) is a global drug delivery technology company that applies proprietary materials science, formulation research and manufacturing innovation to advance the quality, therapeutic value, development speed and rapid market availability of pharmaceuticals, biologics, vaccines and consumer healthcare products. West is the world's premiere provider of standard-setting systems and device components for parenterally administered medicines and an emerging leader in the development of advanced formulation technologies for the transmucosal delivery of drugs. Internationally headquartered in Lionville, Pennsylvania, West supports its partners and customers from 50 locations throughout North America, South America, Europe, Mexico, Japan, Asia and the Pacific and Australia. For more information visit West at www.westpharma.com.
Cautionary Statement Regarding Forward-Looking Information
Certain statements contained in this Report or in other company documents and certain statements that may be made by management of the Company orally may contain forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995. These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as "estimate," "expect," "intend," "believe," "plan, " "anticipate" and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. In particular, these include statements concerning future actions, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
Because actual results are affected by risks and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward-looking statement.
It is not possible to predict or identify all such risks and uncertainties, but factors that could cause the actual results to differ materially from expected and historical results include, but are not limited to: sales demand, timing of customers' projects; successful development of proprietary drug delivery technologies and systems; regulatory, licensee and/or market acceptance of products based on those technologies; competitive pressures; the strength or weakness of the U.S. dollar; inflation; the cost of raw materials; the availability of credit facilities; and, statutory tax rates. With respect to the explosion and fire at the Company's Kinston, NC plant, the following factors should also be taken into consideration: the timely replacement of production capacity; the adequacy and timing of insurance recoveries for property losses; the unpredictability of existing and future possible litigation related to the explosion and the adequacy of insurance recoveries for costs associated with such litigation; government actions or investigations affecting the Company; the ability of the Company to successfully shift production and compounding capacity to other plant sites in a timely manner, including the successful integration of experienced personnel to other production sites; the extent of uninsured costs for, among other things, legal and investigation services and incremental insurance; and regulatory approvals and customer acceptance of goods from alternate sites.
The Company assumes no obligation to update forward-looking statements as circumstances change. Investors are advised, however, to consult any further disclosures the Company makes on related subjects in the Company's 10-K, 10-Q and 8-K reports.
COPYRIGHT 2003 Business Wire
COPYRIGHT 2003 Gale Group