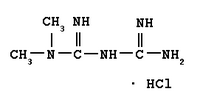
The Cochrane Abstract below is a summary of a review from the Cochrane Library. It is accompanied by an interpretation that will help clinicians put evidence into practice. Melissa Nothnagle, M.D., and Julie Scott Taylor, M.D., M.Sc., present a clinical scenario and question based on the Cochrane Abstract, along with the evidence-based answer and a full critique of the abstract.
This clinical content conforms to AAFP criteria for evidence-based continuing medical education (EB CME). EB CME is clinical content presented with practice recommendations supported by evidence that has been systematically reviewed by an AAFP-approved source. The practice recommendations in this activity are available at http://www.update-software.com/ abstracts/ab003053.htm.
Clinical Scenario
A 30-year-old nulliparous woman who recently was diagnosed with polycystic ovary syndrome wants to become pregnant. Her body mass index (BMI) is 31.4 kg per m2, and her low-density lipoprotein (LDL) cholesterol level is 154 mg per dL (4.0 mmol per L).
Clinical Question
Does metformin therapy improve the clinical features of polycystic ovary syndrome and increase the likelihood of ovulation?
Evidence-Based Answer
Metformin therapy improves fasting insulin levels and blood pressure. It has no clinically significant effect on body weight, waist:hip ratio, or LDL cholesterol level. Metformin, taken with or without clomiphene, appears to be an effective first-line agent for ovulation induction in women with polycystic ovary syndrome. The safety and efficacy of continuing metformin therapy during pregnancy has not been established.
Practice Pointers
Polycystic ovary syndrome is one of the most common endocrinopathies among women of reproductive age. (2) The National Institutes of Health diagnostic criteria define the syndrome as anovulation and hyperandrogenism (clinical signs or elevated hormone levels) in the absence of secondary causes.3 Ultrasonography findings of polycystic ovaries are nonspecific. Polycystic ovary syndrome frequently is associated with hyperinsulinemia and increased risk for type 2 diabetes.
Based on this review, advantages of metformin therapy for patients with polycystic ovary syndrome include clinically significant improvements in insulin resistance and blood pressure. Statistically significant lowering of LDL cholesterol levels was noted in patients taking metformin; however, the effect was too small to be clinically relevant. In addition, the reviewers found increased rates of ovulation in patients with polycystic ovary syndrome who were treated with metformin (compared with placebo) or metformin with clomiphene (compared with clomiphene alone). Dosages of oral metformin used in these studies were 500 mg three times daily or 850 mg twice daily.
Metformin use is associated with significant gastrointestinal side effects, including nausea, vomiting, diarrhea, and abdominal discomfort, which led some participants to withdraw from the reviewed studies. In practice, these side effects can be minimized with gradual dose titration. (4)
Lactic acidosis, a rare but serious adverse event associated with metformin use, did not occur in any of the studies reviewed. Contraindications include risk factors for metformin-associated lactic acidosis, such as congestive heart failure, hepatic insufficiency, impaired renal function (serum creatinine level greater than 1.5 mg per dL [132.6 [micro]mol per L] in men or greater than 1.4 mg per dL [123.8 [micro]mol per L] in women), and any illness characterized by hypoxia or hypoperfusion.
There currently are no long-term data on the effects of metformin use in young, nondiabetic women. Data are limited on metformin use during pregnancy, but there has been no evidence of teratogenicity, (5) and metformin is categorized as a pregnancy category B agent. Because of limited data on first-trimester effects in humans, metformin therapy usually is discontinued when pregnancy is confirmed. However, two small studies of metformin use throughout pregnancy in women with polycystic ovary syndrome showed a reduced risk of spontaneous abortion and gestational diabetes with metformin use. (5,6) Neither study showed an association between the medication and congenital defects or neonatal complications. Compared with metformin use and combination therapy with metformin and clomiphene, increased exercise and weight loss may result in higher ovulation rates in women with polycystic ovary syndrome. (7) Metformin always should be used as an adjuvant to general lifestyle improvements, not as a substitute for diet and exercise.
REFERENCES
(1.) Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev 2003:CD003053.
(2.) Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Encrinol Metab 1998;83:3078-82.
(3.) National Institutes of Health Consensus Meeting on PCOS. In: Dunaif A, ed. Current issues in endocrinology and metabolism. Boston: Blackwell Scientific, 1992.
(4.) Phillips BB. Managing therapy and adverse effects with antihyperglycemic agents: a focus on metformin and acarbose. Pharm Pract Manag Q 1997;17:21-31.
(5.) Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod 2002;17:2858-64
(6.) Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab 2002;87:524-9.
(7.) Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 1998;13:1502-5.
RELATED ARTICLE: Cochrane abstract.
Background. Polycystic ovary syndrome is characterized by anovulation, hyperandrogenemia, and insulin resistance. Hyperinsulinemia is associated with an increase in cardiovascular risk and the development of diabetes mellitus. If insulin-sensitizing agents such as metformin are effective in treating features of polycystic ovary syndrome, they could have health benefits wider than simply treating the symptoms of the syndrome.
Objectives. To assess the effectiveness of insulin-sensitizing drugs in improving clinical and biochemical features of polycystic ovary syndrome.
Search Strategy. The authors1 searched the Cochrane Menstrual Disorders and Subfertility Group trials register (December 2002), the Cochrane Central Register of Controlled Trials (Cochrane Library, Issue 4, 2002), MEDLINE (January 1966 to December 2002), and EMBASE (January 1985 to December 2002).
Selection Criteria. Randomized controlled trials that investigated the effects of insulin-sensitizing drugs compared with placebo or no treatment, or insulin-sensitizing drugs compared with an ovulation-induction agent.
Data Collection and Analysis. The analysis was performed by two reviewers, one blinded to information that could have identified the authors, publisher, or results of each study. Fifteen trials were included for analysis, 13 of them using metformin, and involving 543 participants.
Primary Results. Meta-analysis showed that metformin is effective in achieving ovulation in women with polycystic ovary syndrome, with odds ratios of 3.88 (95 percent confidence interval [CI], 2.25 to 6.69) for metformin versus placebo and 4.41 (95 percent CI, 2.37 to 8.22) for metformin and clomiphene versus clomiphene alone. An analysis of pregnancy rates suggests a significant treatment effect for metformin and clomiphene (odds ratio, 4.40; 95 percent CI, 1.96 to 9.85). Metformin has a significant effect in reducing fasting insulin levels (weighted mean difference, -5.37; 95 percent CI, -8.11 to -2.63), blood pressure, and LDL cholesterol level. There was no evidence of effect on BMI or waist:hip ratio. Metformin was associated with a significantly higher incidence of nausea, vomiting, and other gastrointestinal disturbance, but no serious adverse effects were reported.
Reviewers' Conclusions. Metformin is an effective treatment for anovulation in women with polycystic ovary syndrome. Its choice as a first-line agent seems justified, and there is some evidence of benefit on parameters of the metabolic syndrome. Ovulation rates are higher when combined with clomiphene (76 percent versus 46 percent when used alone), but there is no evidence to indicate whether there is an increased multiple pregnancy rate with this combination. There are no data regarding its safety in long-term use in young women. It should be used as an adjuvant to general lifestyle improvements, not as a replacement for increased exercise and improved diet.
Melissa Nothnagle, M.D., is clinical assistant professor of family medicine at Brown Medical School, Pawtucket, R.I. Julie Scott Taylor, M.D., M.Sc., is assistant professor of family medicine and director of predoctoral education at Brown Medical School.
Address correspondence to Melissa Nothnagle, M.D., Department of Family Medicine, Memorial Hospital of Rhode Island, 111 Brewster St., Pawtucket, RI 02860 (e-mail: Melissa_Nothnagle@mhri.org). Reprints are not available from the authors.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group