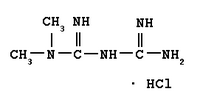
Polycystic ovary syndrome (PCOS), a combination of oligomenorrhea or anovulation with hyperandrogenism, affects approximately 5 to 7 percent of women of reproductive age. This neuroendocrine abnormality may be caused by elevated luteinizing hormone (LH) levels and more frequent pulses of LH secretion. Women with hyperandrogenism also have increased insulin resistance, which manifests as increased body mass index (BMI), increased waist-to-hip ratio or, in severe cases, acanthosis nigricans. The combination of severe insulin resistance and LH stimulation results in increased ovarian secretion of testosterone, leading to the virilizing features of PCOS. The syndrome should be suspected in women with hirsutism, irregular menstruation, or infertility. Traditional treatment involves the use of clomiphene therapy and weight loss for subfertility, and combination oral contraceptives plus spironolactone for hirsutism. Barbieri conducted a review of the increasing role of insulin sensitizers, such as metformin, in the effective treatment of women with PCOS.
From more than 90 articles on metformin and PCOS, the author identified 21 studies that reported clinical outcomes or significant aspects of the clinical pharmacology of metformin related to PCOS. Metformin does not produce hypoglycemia but is thought to act on several cellular processes, including hepatic glucose production, intestinal absorption of glucose, gluconeogenesis, and the use of insulin-mediated glucose in peripheral tissues.
In one large clinical trial of 3,234 adults with elevated fasting and postglucose-load plasma glucose concentrations, metformin reduced the risk of progression to diabetes by 31 percent compared with placebo. Even greater reductions were achieved with the addition of aggressive lifestyle modifications. The author emphasizes the need for both lifestyle changes and metformin therapy to reduce the risk of diabetes in women with PCOS.
Metformin also is effective in achieving weight loss in women with PCOS. It potentiates the low-calorie diets typically used to achieve the BMI of 20 to 25 kg per m2 that is necessary for the return of ovulation. In one study of 150 obese women, a 10 percent reduction in BMI was achieved with metformin therapy. In another study, metformin plus a low-calorie diet was superior to the low-calorie diet alone for weight loss in women with PCOS. The weight loss action of metformin appears to be caused by the reduction in insulin resistance as well as by appetite suppression. Metformin is the only antidiabetic agent associated with weight loss rather than weight gain and is, thus, particularly suitable for therapy in patients with PCOS.
The effects of metformin on menstrual function and infertility may be caused by decreased insulin resistance and lowered testosterone levels. In a long-term study of 23 women with PCOS, one half of those treated with metformin resumed regular menstruation. The effect appears to increase with duration of treatment. Studies of women who were treated for at least six months report that more than 90 percent of women resumed regular menstruation. Four to six months of therapy are thought to be necessary for ovulation to commence. The effect of metformin on hirsutism has not been extensively reported, and androgen-blocking drugs may be more effective than metformin for treatment of this symptom.
In studies, about 5 percent of patients discontinued metformin therapy because of side effects, but more than one half of patients reported diarrhea, and one fourth experienced other gastrointestinal upsets. A rare but potentially serious side effect is lactic acidosis in patients with renal insufficiency. Metformin also may interact with medications such as cimetidine, digoxin, amiloride, quinidine, vancomycin, and trimethoprim, and may interfere with vitamin B12 absorption.
The author concludes that metformin in dosages of 1,500 to 2,550 mg per day addresses the major aspects of PCOS management and is expected to become more widely used to treat this syndrome. Because of the gastrointestinal side effects of metformin, the usual starting dosage is 500 mg taken with the largest meal of the day. If tolerated, the dosage is gradually increased to 500 mg with each meal. Clinical effect does not usually occur at dosages of less than 1,000 mg per day, and the optimal effect may not be apparent for several months.
Anne D. Walling, M.D.
Barbieri RL. Metformin for the treatment of polycystic ovary syndrome. Obstet Gynecol April 2003;101;785-93.
COPYRIGHT 2003 American Academy of Family Physicians
COPYRIGHT 2003 Gale Group