Definition
Lipidoses are heredity disorders, passed from parents to their children, characterized by defects of the digestive system that impair the way the body uses fat from the diet. When the body is unable to properly digest fats, lipids accumulate in body tissues in abnormal amounts.
Description
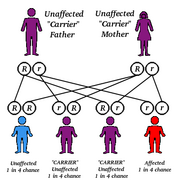
The digestion, storage, and use of fats from foods is a complex process that involves hundreds of chemical reactions in the body. In most people, the body is already programmed by its genetic code to produce all of the enzymes and chemicals necessary to carry out these functions. These genetic instructions are passed from parents to their offspring during reproduction.
People with lipidoses are born without the genetic codes needed to tell their bodies how to complete a particular part of the fat digestion process. In most of these disorders, the body does not produce a certain enzyme or chemical. Over 30 different disorders of fat metabolism are related to genetic defects. Although the defects are passed from parents to children, the parents often do not have the disorders themselves.
The symptoms, available treatments, and long-term consequences of these conditions vary greatly. Some of the conditions become apparent shortly after the infant is born; in others, symptoms may not develop until adulthood. For most of the lipidoses, diagnosis is suspected based on the symptoms and family history. Blood tests, urine tests, and tissue tests can be used to confirm the diagnosis. Genetic testing can be used, in some cases, to identify the defective gene. Some of these disorders can be controlled with changes in the diet, medications, or enzyme supplements. For many, no treatment is available. Some may cause death in childhood or contribute to a shortened life expectancy. Some of the most common or most serious lipidoses are discussed below.
fabry's disease
Causes & symptoms
Approximately 1 in every 40,000 males is born with Fabry's disease. This condition has an X-linked, recessive pattern of inheritance, meaning that the defective gene is carried on the X chromosome. A female who carries a defective recessive gene on one of her two X chromosomes has a 50% chance of passing the defective gene to her sons who will develop the disorder associated with the defective gene (a male receives one X chromosome from his mother and one Y chromosome from his father). She also has a 50% chance of passing the defective recessive gene to her daughters who will be carries of the disorder (like their mother). Some female carries of Fabry's disease show mild signs of the disorder, especially cloudiness of the cornea.
The gene that is defective in Fabry's disease causes a deficiency of the enzyme alpha-galactosidase A. Without this enzyme, fatty compounds starts to line the blood vessels. The collection of fatty deposits eventually affects blood vessels in the skin, heart, kidneys, and nervous system. The first symptoms in childhood are pain and discomfort in the hands and feet brought on by exercise, fever, stress, or changes in the weather. A raised rash of dark red-purple spots is common, especially on skin between the waistline and the knees. Other symptoms include a decreased ability to sweat and changes in the cornea or outer layer of the eye. Although the disease begins in childhood, it progresses very slowly. Kidney and heart problems develop in adulthood.
Diagnosis
The diagnosis can be confirmed by a blood test to measure for alpha-galactosidase A. Women who are carries of the defective gene can also be identified by a blood test.
Treatment
Treatment focuses on prevention of symptoms and long-term complications. Daily doses of diphenylhydantoin (Dilantin) or carbamazapine (Tegretol) can prevent or reduce the severity of pain in the hands and feet associated with the condition. A low sodium, low protein diet may be beneficial to those patients who have some kidney complications. If kidney problems progress, kidney dialysis or kidney transplantation may be required. Enzyme replacement therapy is currently being explored.
Prognosis
Although patients with Fabry's disease usually survive to adulthood, they are at increased risk for stroke, heart attacks, and kidney damage.
gaucher disease
Causes & symptoms
Gaucher (pronounced go-shay) disease is the most common of the lipid storage disorders. It is found in populations all over the world (20,00 to 40,000 people have a type of the disease), and it occurs with equal frequency in males and females. Gaucher disease has a recessive pattern of inheritance, meaning that a person must inherit a copy of the defective gene from both parents in order to have the disease. The genetic defect causes a deficiency of the enzyme glucocerebrosidase that is responsible for breaking down a certain type of fat and releasing it from fat cells. These fat cells begin to crowd out healthy cells in the liver, spleen, bones, and nervous system. Symptoms of Gaucher disease can start in infancy, childhood, or adulthood.
Three types of Gaucher disease have been identified, but there are many variations in how symptoms develop. Type 1 is the most common and affects both children and adults. It occurs much more often in people of Eastern European and Russian Jewish (Ashkenazi) ancestry, affecting 1 out of every 450 live births. The first signs of the disease include an enlarged liver and spleen, causing the abdomen to swell. Children with this condition may be shorter than normal. Other symptoms include tiredness, pain, bone deterioration, broken bones, anemia, and increased bruising. Type 2 Gaucher disease is more serious, beginning within the first few months after birth. Symptoms, which are similar to those in Type 1, progress rapidly, but also include nervous system damage. Symptoms of Type 3 Gaucher disease begin during early childhood with symptoms like Type 1. Unlike Type 2, the progress of the disease is slower, although it also includes nervous system damage.
Diagnosis
Gaucher disease may be suspected based on symptoms and is confirmed with a blood test for levels of the enzyme. Samples of tissue from an affected area may also be used to confirm a diagnosis of the disease.
Treatment
The symptoms of Gaucher disease can be stopped and even reversed by treatment with injections of enzyme replacements. Two enzyme drugs currently available are alglucerase (Ceredase) and imiglucerase (Cerezyme). Other treatments address specific symptoms such as anemia, broken bones, or pain.
Prognosis
The pain and deformities associated with symptoms can make coping with this illness very challenging for individuals and families. With treatment and control of symptoms, people with Type 1 Gaucher disease may lead fairly long and normal lives. Most infants with Type 2 die before the age of 2. Children with Type 3 Gaucher disease may survive to adolescence and early adulthood.
krabbe's disease
Causes & symptoms
Krabbe's disease is caused by a deficiency of the enzyme galactoside beta-galactosidase. It has a recessive pattern of inheritance and is believed to occur in 1 of 40,000 births in the United States. This condition, which is also called globoid cell leukodystrophy or Krabbe leukodystrophy, is characterized by acute nervous system degeneration. It develops in early infancy with initial symptoms of irritability, vomiting and episodes of partial unconsciousness. Symptoms progress rapidly to seizures, difficulty swallowing, blindness, deafness, mental retardation, and paralysis.
Treatment
No treatment is available.
Prognosis
Children born with Krabbe's disease die in infancy.
niemann-pick disease
Causes & symptoms
At least five different forms of Niemann-Pick disease (NPD) have been identified. The different types seem to be related to the activity level of the enzyme sphingomyelinase. In patients with Types A and B NPD, there is a build up of sphingomyelin in cells of the brain, liver, spleen, kidney and lung. Type A is the most common form of NPD and the most serious, with death usually occurring by the age of 18 months. Symptoms develop within the first few months of life and include poor appetite, failure to grow, enlarged liver and spleen, and the appearance of cherry red spots in the retina of the eye. Type B develops in infancy or childhood with symptoms of mild liver or spleen enlargement and lung problems. Some adults with this form (Type E) may also show a loss of muscle coordination. Types C or D NPD are related to cholesterol transfer out of cells. Children with Types C or D grow normally in early childhood, but eventually develop difficulty in walking and loss of muscle coordination. Ultimately, the nervous system becomes severely damaged and these patients die. Type C occurs in any population, while Type D has been identified only in patients from Nova Scotia, Canada.
Diagnosis
Diagnosis is confirmed by analyzing a sample of tissue. Prenatal diagnosis of Types A and B of NPD can be done with amniocentesis or chorionic villus sampling.
Treatment
Treatment consists of supportive care to deal with symptoms and the development of complications. Bone marrow transplantation is being investigated as a possible treatment. Low-cholesterol diets may be helpful for patients with Types C and D.
Prognosis
Patients with Type A NPD usually die within the first year and a half of life. Type B patients generally live to adulthood but suffer from significant liver and lung problems. With Types C and D NPD, there is significant nervous system damage leading to severe muscle spasms, seizures, and eventually, to coma and death. Some patients with Types C and D die in childhood, while less severely affected patients may survive to adulthood.
refsum's disease
Causes & symptoms
Refsum's disease has a recessive pattern of inheritance and affects populations from Northern Europe, particularly Scandinavians most frequently. It is due to a deficiency of phytanic acid hydroxylase, an enzyme that breaks down a fatty acid called phytanic acid. This condition affects the nervous system, eyes, bones, and skin. Symptoms, which usually appear by age 20, include vision problems [retinitis pigmentosa and rhythmic eye movements (nystagmus)], loss of muscle coordination, loss of sense of smell (anosmia), pain, numbness, and elevated protein in the cerebrospinal fluid.
Treatment
A diet free of phytanic acid (found in dairy products, tuna, cod, haddock, lamb, stewed beef, white bread, white rice, boiled potatoes, and egg yolk) can reduce some of the symptoms. Plasmapheresis, a process where whole blood is removed from the body, processed through a filtering system, and then return to the body, may be used to filter phytanic acid from the blood.
tay-sachs disease
Causes & symptoms
Tay-Sachs disease (TSD) is a fatal condition caused by a deficiency of the enzyme hexosaminidase A (Hex-A). The defective gene that causes this disorder is found in roughly 1 in 250 people in the general population. However, certain populations have significantly higher rates of TSD. French-Canadians living near the St. Lawrence River and in the Cajun regions of Louisiana are at higher risk of having a child with TSD. The highest risk seems to be in people of Eastern European and Russian Jewish (Ashkenazi) descent. Tay-Sachs disease has a recessive pattern of inheritance, and approximately 1 in every 27 people of Jewish ancestry in the United States carries the TSD gene. Symptoms develop in infancy and are due to the accumulation of a fatty acid compound in the nervous system. Early symptoms include loss of vision and physical coordination, seizures, and mental retardation. Eventually, the child develops problems with breathing and swallowing. Blindness, paralysis, and death follow.
Diagnosis
Carriers of the Tay-Sachs related gene can be identified with a blood test. Amniocentesis or chorionic villi sampling can be used to determine if the fetus has Tay-Sachs disease.
Treatment
There is no treatment for Tay-Sachs disease. Parents who are identified as carriers may want to seek genetic counseling. If a fetus is identified as having TSD, parents may consider termination of the pregnancy.
Prognosis
Children born with Tay-Sachs disease become increasingly debilitated; most die by about age four.
wolman's disease
Causes & symptoms
Wolman's disease is caused by a genetic defect (with a recessive pattern of inheritance) that results in deficiency of an enzyme that breaks down cholesterol. This causes large amounts of fat to accumulate in body tissues. Symptoms begin in the first few weeks of life and include an enlarged liver and spleen, adrenal calcification (hardening of adrenal tissue due to deposits of calcium salts), and fatty stools.
Treatment
No treatment is currently available.
Prognosis
Death generally occurs before six months of age.
Prevention
Couples who have family histories of genetic defects can undergo genetic testing and counseling to see if they are at risk for having a child with one of the lipidoses disorders. During pregnancy, cell samples can be collected from the fetus using amniocentesis or chorionic villi sampling. The results of these test can indicate if the developing fetus has a lipidosis disorder. Termination of the pregnancy may be considered in some cases.
Key Terms
- Amniocentesis
- A procedure where a needle is inserted through the abdomen into the uterus of a pregnant woman to remove a small amount of the fluid that surrounds the developing fetus. This test can be preformed at about week 16 of the pregnancy. Cells from the fetus can be tested for genetic defects.
- Chorionic villi sampling
- A procedure to remove a small tissue sample of the placenta, the sac that surrounds the developing fetus. This test can be performed as early as week 10 of the pregnancy. The tissue can be tested for genetic defects.
- Lipids
- Organic compounds not soluble in water, but soluble in fat solvents such as alcohol. Lipids are stored in the body as energy reserves and are also important components of cell membranes.
- Recessive
- Refers to an inherited characteristic or trait that is expressed only when two copies of the gene responsible for it are present.
- X-linked
- Refers to a gene carried on the X chromosome, one of the two sex chromosomes.
Further Reading
For Your Information
Books
- "Lipid Storage Diseases." In Internal Medicine, edited by Jay H. Stein. 5th ed. St. Louis: Mosby, 1998.
- McGovern, Margaret M., and Robert J. Desnick. "Lysosomal Storage Diseases." In Cecil Textbook of Medicine, edited by J. Claude Bennett and Fred Plum. 20th ed. Philadelphia: W.B. Saunders, 1996.
- Valle, David L., ed. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. New York: McGraw-Hill, 1994.
Organizations
- International Center for Fabry Disease. Department of Human Genetics, Mount Sinai School of Medicine, Box 1497, Fifth Avenue and 100th Street, New York, NY 10029. (212) 241-6944. http://www.mssm.edu/crc/fabry/brochure.html.
- National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), 9000 Rockville Pike, Bethesda, MD 20892. (301) 496-3583.
- National Institutes of Health. National Institute of Neurological Disorders and Stroke, 9000 Rockville Pike, Bethesda, MD 20892. (301) 496-5751. (800) 352-9424.
- National Niemann-Pick Foundation. 3734 E. Olive Avenue, Gilbert, AZ 85234. (602) 497-6638.
- National Organization for Rare Disorders (NORD). PO Box 8923, New Fairfield, CT 06812-8923. (203) 746-6518. (800) 999-6673.
- National Tay-Sachs and Allied Diseases Association, Inc. 2001 Beacon Street, Brookline, MA 02146. (617) 277-4463. (800) 906-8723. http://mcrcr2.med.nyu.edu/murphp01/ntsad/t-sachs.htm.
Other
- Gaucher Disease Treatment Program at Massachusetts General Hospital. http://neuro-www2.mgh.harvard.edu/gaucher/main.html.
- Rare Genetic Diseases In Children: An Internet Resource Gateway. http://mcrcr2.med.nyu.edu/murphp01/homenew.htm.
Gale Encyclopedia of Medicine. Gale Research, 1999.